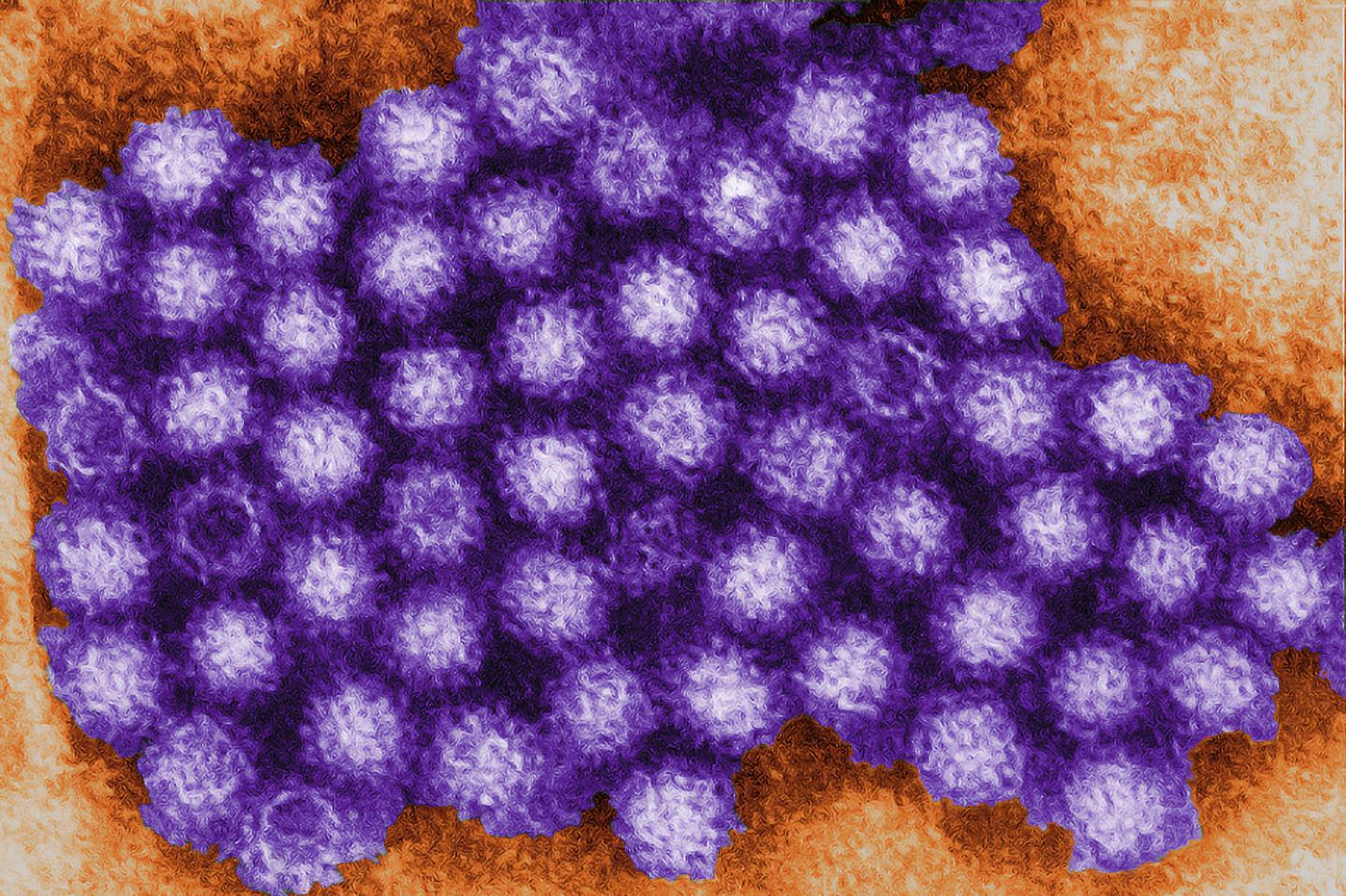
Norovirus is the most common viral cause of diarrhea worldwide, but scientists still know little about how it infects people and causes disease. Research has been hindered by an inability to grow the virus in the lab.
Now, researchers at Washington University School of Medicine in St. Louis have identified the protein that norovirus uses to invade cells. The discovery, in mice, provides new ways to study a virus notoriously hard to work with and may lead to treatments or a vaccine.
"Our inability to grow the virus in the lab has limited our ability to develop anti-viral agents. If you can't get the virus to multiply in human cells, how are you going to find compounds that inhibit multiplication?" said Herbert "Skip" Virgin, MD, PhD, the Mallinckrodt Professor and Chair of the Department of Pathology and Immunology and the study's senior author. "This discovery provides a good basis for our mouse model, which we can then use to understand noroviral pathogenesis and search for treatments in people."
The research is published August 18 in Science.
Norovirus is infamous for causing outbreaks of diarrhea, vomiting and stomach cramps on cruise ships, in military barracks and in other environments where people live in close quarters. For most people, infection leads to an uncomfortable day or two punctuated with frequent trips to the bathroom, but in vulnerable populations such as cancer patients and older people, the disease can be long-lasting and sometimes deadly.
There are many noroviruses, but each is restricted to infecting just one animal species. Human norovirus will not infect any of the species typically used in biomedical research, such as mice, rats or rabbits. Human norovirus won't grow even in human cells in petri dishes.
"Since human norovirus won't grow in human cell lines or laboratory animals, you can't test a drug, you can't test a vaccine," Virgin said. "You'd have to do those kinds of studies in people, but it would be better if we can first conduct tests in animal models."
When mouse norovirus was discovered in 2003, it seemed like a great opportunity to make a mouse model of norovirus infection. The genomes of mouse and human norovirus are very similar, and the viruses even look alike under the electron microscope. Nobody could ever be sure, however, that how mouse norovirus acts in mice is relevant to how human norovirus acts in humans.
Virgin and postdoctoral researchers Craig Wilen, MD, PhD, and Robert Orchard, PhD, thought that if they could identify the reason that mouse norovirus infects only mice and human norovirus infects only humans, they could improve their model of norovirus infection.
The researchers used a genetic tool known as CRISPR-Cas9 to identify mouse genes that are important for mouse noroviral infection. They found that when a gene called CD300lf was knocked down by CRISPR-Cas9, norovirus could not infect the cells. CD300lf codes for a protein on the surface of mouse cells, and the researchers believe the virus latches on to it to get inside the cell.
Furthermore, when the researchers expressed mouse CD300lf protein on the surface of human cells, mouse norovirus was able to infect the human cells and multiply. "Mouse norovirus grew just fine in human cells," Virgin said. "This tells us that the species restriction is due to the ability to get inside the cells in the first place. Once inside the cells, most likely all the other mechanisms are conserved between human and mouse noroviruses, since the viruses are so similar."
The researchers also found that mouse norovirus requires a second molecule, or cofactor, to infect cells; CD300lf by itself isn't enough. But they were unable to nail down the molecule's identity.
"At this point we know more about what it isn't than what it is," said Orchard, a co-lead author on the study. "Every week there's a new favorite hypothesis. It's probably a small molecule found in the blood, not a protein."
It is unusual for a virus to require a cofactor for infection. Their discovery suggests that the lack of a necessary cofactor may be why scientists have had a difficult time growing human norovirus in the lab.
The researchers are working on ways to use human cells with the mouse CD300lf protein to study noroviral infection. One possibility is to use the system to screen drugs to block viral multiplication. Such drugs could be administered prophylactically to people around the epicenter of an outbreak, or as a treatment for immunocompromised individuals.
The discovery of the mouse receptor for norovirus also could lead to a better understanding of how the virus causes disease.
"We still don't even know if the virus infects epithelial cells or immune cells, and that matters if you want to develop a vaccine," said Wilen, a co-lead author on the study. "We have developed a knockout mouse that lacks CD300lf, and we are using it to identify the cell types involved. We're hoping that a better understanding of the pathogenesis will lead to better ways to treat or prevent this very common disease."
###
Media Contact
Judy Martin Finch
[email protected]
314-286-0105
@WUSTLmed
Home