Credit: ZHUANG Taotao
Liquid multi-carbon alcohols such as ethanol and n-propanol are desired as renewable transportation fuels. They offer high energy densities, ease of long-range transport, and direct drop-in usage in existing internal combustion engines. Engineering catalysts that favor high-value alcohols is desired.
A research team led by professor YU Shuhong from University of Science and Technology of China of Chinese Academy of Sciences and Edward H. Sargent from University of Toronto has uncovered a catalysis strategy intermediates during CO2 electrochemical reduction reaction, which sheds new lights on upgrading CO2 to engine fuels.
When it comes to designing catalysts for CO2 conversion, many researches on the C-C coupling step have been done; while little attention has been paid to post-C-C coupling reaction.
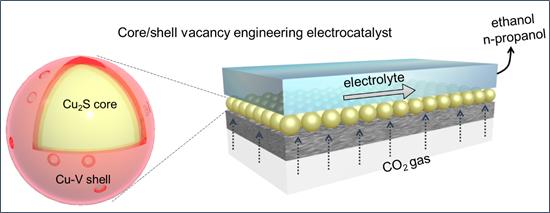
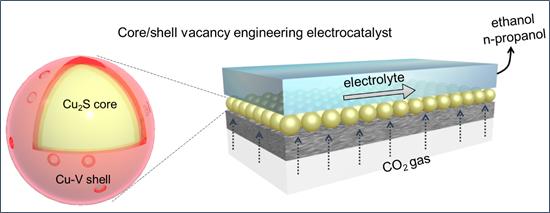
By deliberately incorporating sulfur atoms into the catalyst core and copper vacancies in its shell, researchers realized Cu2S-Cu-V core-shell nanoparticles that enhance CO2 reduction to ethanol and propanol. Structural characterization, x-ray studies, and electrochemical measurements were utilized to illustrate how good this new catalyst is in improving catalytic performance.
This discovery will inspire the design of efficient catalysts that produce higher-carbon liquid alcohols. In addition to address the need for long-term storage of renewable electricity and decarbonization of the transportation sector via electrocatalytic reduction of CO2 to chemical feedstocks. And the results were published in Nature Catalysis on Jun 11th.
###
This work was supported by Foundation for Innovative Research Groups of the National Natural Science Foundation of China, the National Natural Science Foundation of China and other foundations and institutions.
Media Contact
FAN Qiong
[email protected]
86-636-07280
http://en.ustc.edu.cn
Related Journal Article
http://dx.doi.org/10.1038/s41929-018-0084-7