Credit: Cell Stem Cell/the Park lab
Led by researchers at Baylor College of Medicine, a study published in the journal Cell Stem Cell reveals a new mechanism that contributes to adult bone maintenance and repair and opens the possibility of developing therapeutic strategies for improving bone healing.
“Adult bone repair relies on the activation of bone stem cells, which still remain poorly characterized,” said corresponding author Dr. Dongsu Park, assistant professor of molecular and human genetics and of pathology and immunology at Baylor. “Bone stem cells have been found both in the bone marrow inside the bone and also in the periosteum – the outer layer of tissue – that envelopes the bone. Previous studies have shown that these two populations of stem cells, although they share many characteristics, also have unique functions and specific regulatory mechanisms.”
Of the two, periosteal stem cells are the least understood. It is known that they comprise a heterogeneous population of cells that can contribute to bone thickness, shaping and fracture repair, but scientists had not been able to distinguish between different subtypes of bone stem cells to study how their different functions are regulated.
In the current study, Park and his colleagues developed a method to identify different subpopulations of periosteal stem cells, define their contribution to bone fracture repair in live mouse models and identify specific factors that regulate their migration and proliferation under physiological conditions.
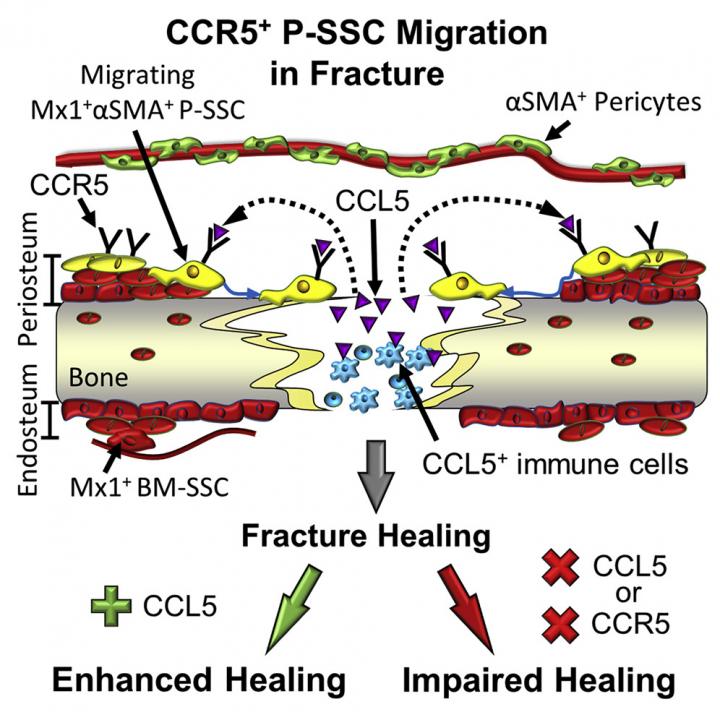
Periosteal stem cells are major contributors to bone healing
The researchers discovered specific markers for periosteal stem cells in mouse models. The markers identified a distinct subset of stem cells that contributes to life-long adult bone regeneration.
“We also found that periosteal stem cells respond to mechanical injury by engaging in bone healing,” Park said. “They are important for healing bone fractures in the adult mice and, interestingly, their contribution to bone regeneration is higher than that of bone marrow stem cells.”
In addition, the researchers found that periosteal stem cells also respond to inflammatory molecules called chemokines, which are usually produced during bone injury. In particular, they responded to chemokine CCL5.
Periosteal stem cells have receptors – molecules on their cell surface – that bind to CCL5, which sends a signal to the cells to migrate toward the injured bone and repair it. Deleting the CCL5 gene in mouse models resulted in marked defects in bone repair or delayed healing. When the researchers supplied CCL5 to CCL5-deficient mice, bone healing was accelerated.
The findings suggested potential therapeutic applications. For instance, in individuals with diabetes or osteoporosis in which bone healing is slow and may lead to other complications resulting from limited mobility, accelerating bone healing may reduce hospital stay and improve prognosis.
“Our findings contribute to a better understanding of how adult bones heal. We think this is one of the first studies to show that bone stem cells are heterogeneous and that different subtypes have unique properties regulated by specific mechanisms,” Park said. “We have identified markers that enable us to tell bone stem cell subtypes apart and studied what each subtype contributes to bone health. Understanding how bone stem cell functions are regulated offers the possibility to develop novel therapeutic strategies to treat adult bone injuries.”
###
Other contributors to this work include Laura C. Ortinau, Hamilton Wang, Kevin Lei, Lorenzo Deveza, Youngjae Jeong, Yannis Hara, Ingo Grafe, Scott Rosenfeld, Dongjun Lee, Brendan Lee and David T. Scadden. The authors are affiliated with one of the following institutions: Baylor College of Medicine, Texas Children’s Hospital, Pusan National University School of Medicine and Harvard University.
This study was supported by the Bone Disease Program of Texas Award and the Caroline
Wiess Law Fund Award, the NIAMS of the National Institutes of Health under award numbers 1K01AR061434 and 1R01AR072018 and U54 AR068069 and the NIDDK of the NIH.
Media Contact
Molly Chiu
[email protected]
713-798-4710
Original Source
https:/
Related Journal Article
http://dx.




