Nowadays, hepatocellular carcinoma (HCC) is the third most common cause of cancer-related deaths worldwide. For most intermediate-advanced HCC patients, transarterial embolization (TAE) therapy is the mainstream treatment by utilizing embolic agents to block the tumor blood supply to induce ischemic necrosis attributing to its minimal invasiveness. In clinical treatment, transarterial chemoembolization (TACE), which combines embolic agents with chemotherapy drugs, is also frequently adopted to achieve improved therapeutic benefit. Among these clinically used embolic agent, Lipiodol is a commonly used liquid embolic agent for TAE treatment, and it has also been collectively applied with chemotherapeutic drugs for TACE. However, the limited stability of such Lipiodol-drug emulsion always led to fast diffusion of drugs from the embolization site, which thereby remarkably weakens the therapeutic efficacy of these chemotherapeutic drugs and imposes systemic toxicity. Therefore, development of stable Lipiodol-drug emulsion with sustainable drug release profiles holds great promise to approach improved treatment of HCCs.
Credit: ©Science China Press
Nowadays, hepatocellular carcinoma (HCC) is the third most common cause of cancer-related deaths worldwide. For most intermediate-advanced HCC patients, transarterial embolization (TAE) therapy is the mainstream treatment by utilizing embolic agents to block the tumor blood supply to induce ischemic necrosis attributing to its minimal invasiveness. In clinical treatment, transarterial chemoembolization (TACE), which combines embolic agents with chemotherapy drugs, is also frequently adopted to achieve improved therapeutic benefit. Among these clinically used embolic agent, Lipiodol is a commonly used liquid embolic agent for TAE treatment, and it has also been collectively applied with chemotherapeutic drugs for TACE. However, the limited stability of such Lipiodol-drug emulsion always led to fast diffusion of drugs from the embolization site, which thereby remarkably weakens the therapeutic efficacy of these chemotherapeutic drugs and imposes systemic toxicity. Therefore, development of stable Lipiodol-drug emulsion with sustainable drug release profiles holds great promise to approach improved treatment of HCCs.
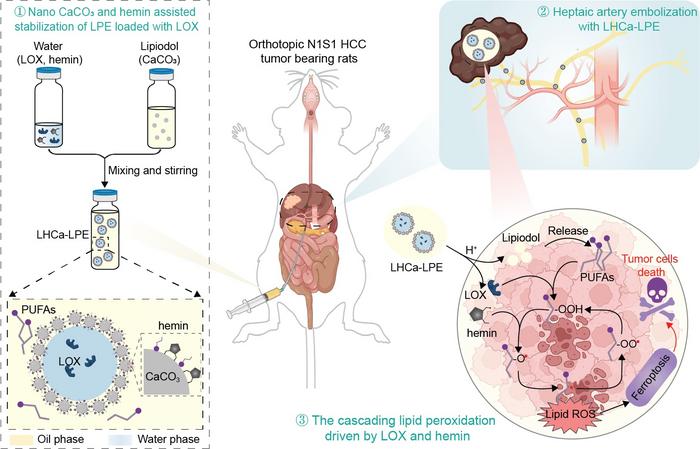
To solve this challenging problem, in a recent research article published in National Science Review, a collaborative research team led by Professor Zhuang Liu from Soochow University (Institute of Functional Nano & Soft Materials, FUNSOM) proposed a water-in-oil Lipiodol Pickering emulsion stabilized by calcium carbonate nanoparticles and hemin. Compared with conventional Lipiodol emulsion, the obtained Lipiodol Pickering emulsion enabled stable encapsulation of various hydrophilic molecules in the aqueous droplets and pH-responsive release of the encapsulated molecules due the presence of CaCO3 nanoparticles. Inspired by the capacity of lipoxygenase (LOX) in promoting the generation of cytotoxic lipid radicals from polyunsaturated fatty acidy, a major component of Lipiodol, a pH-responsive and self-fueling ferroptosis-inducing microreactor (coined as LHCa-LPE) was concisely constructed by encapsulating LOX with such Lipiodol based Pickering emulsion. Such LHCa-LPE was shown to be capable of effectively inducing ferroptosis of cancer cells with Lipiodol as the source of PUFAs via the cascading lipid peroxidation chain reaction. Upon transarterial embolization, such LHCa-LPE could effectively suppress the growth of orthotopic N1S1 HCC in rats by serving as bifunctional embolic and ferroptosis-inducing agents. Overall, this study highlights a facile strategy to prepare stable Lipiodol based embolic agent, which is also promising for potential clinical translation because all components in such emulsions have excellent biocompatibility, holding great promise for clinical translation.
###
See the article:
Self-fueling ferroptosis-inducing microreactors based on pH-responsive Lipiodol Pickering emulsions enable transarterial ferro-embolization therapy
https://doi.org/10.1093/nsr/nwad257
Journal
National Science Review
DOI
10.1093/nsr/nwad257