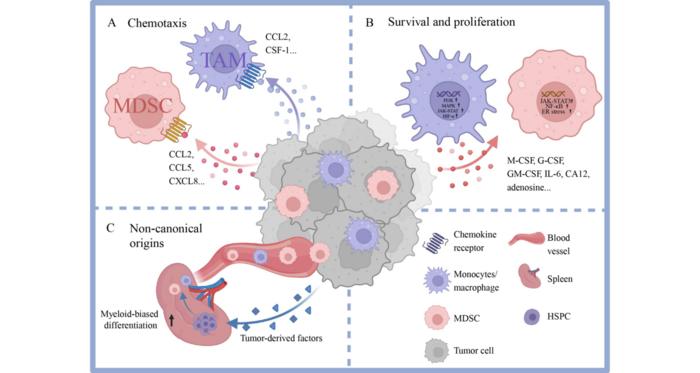
The tumor microenvironment (TME) represents a highly complex and dynamic ecosystem where malignant cells coexist and interact with a diverse array of stromal and immune constituents. Among the immune populations, myeloid cells, particularly tumor-associated macrophages (TAMs) and myeloid-derived suppressor cells (MDSCs), have emerged as pivotal modulators of cancer progression and therapeutic resistance. These cells exhibit remarkable plasticity, adopting distinct functional states according to contextual cues from the microenvironment, including tumor-derived signals, metabolic stressors, and cellular cross-talk. An in-depth understanding of the multifaceted roles and regulatory mechanisms of myeloid cells in the TME holds critical implications for developing next-generation immunotherapies.
TAMs and MDSCs derive primarily from circulating monocytes and bone marrow progenitors, yet recent evidence highlights the contribution of extramedullary hematopoiesis, especially from splenic hematopoietic stem and progenitor cells (HSPCs), to the continuous replenishment of these populations within tumors. This systemic mobilization is driven by tumor-secreted factors that induce emergency myelopoiesis, skewing progenitor differentiation toward immunosuppressive myeloid phenotypes. Once recruited, these cells colonize the tumor stroma, where chemokines such as CCL2, CXCL8, and colony-stimulating factor 1 (CSF-1) orchestrate their accumulation, survival, and proliferation through engagement of receptors like CCR2, CXCR2, and CSF-1R.
The metabolic landscape within the TME imposes significant constraints, including hypoxia and nutrient scarcity, compelling myeloid cells to undergo extensive metabolic reprogramming to sustain their functions. TAMs often upregulate glycolytic pathways, with lactate produced via enhanced glycolysis functioning as an immunomodulatory metabolite that stabilizes hypoxia-inducible factor 1-alpha (HIF-1α). This stabilization promotes epigenetic reprogramming through histone lactylation, driving the transcription of immunosuppressive genes such as interleukin-10 (IL-10) and arginase-1 (Arg1). Concurrently, shifts toward fatty acid oxidation (FAO) and amino acid metabolism are prevalent, with lipid accumulation in TAMs inducing chemokine secretion (e.g., CCL20) that attracts regulatory T cells (Tregs), further reinforcing immune evasion.
.adsslot_iBvjMWUfyC{width:728px !important;height:90px !important;}
@media(max-width:1199px){ .adsslot_iBvjMWUfyC{width:468px !important;height:60px !important;}
}
@media(max-width:767px){ .adsslot_iBvjMWUfyC{width:320px !important;height:50px !important;}
}
ADVERTISEMENT
MDSCs exploit amino acid metabolism, particularly through the catabolism of arginine and tryptophan, to impair effector T cell function. Enzymes such as arginase and indoleamine 2,3-dioxygenase (IDO) contribute to the depletion of these critical nutrients, thus blunting anti-tumor immune responses. Moreover, MDSCs themselves rely heavily on glutamine and FAO pathways for energy and survival, processes intricately regulated by tumor-derived exosomes and cytokines, creating a feedback loop that perpetuates their suppressive capacity. The nuanced interplay between cellular metabolism and immunosuppressive signaling underscores metabolic reprogramming as a central axis dictating myeloid cell function in cancer.
Intracellular signaling cascades governing myeloid cell biology in the TME include activation of transcription factors such as signal transducer and activator of transcription 3 (STAT3) and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB). These pathways are potentiated under hypoxic and metabolic stress conditions, augmenting myeloid survival and facilitating a phenotype conducive to tumor promotion. Additionally, autophagy modulates the cellular homeostasis and plasticity of TAMs and MDSCs, supporting their adaptation to hostile microenvironmental niches. The integration of these diverse signals ensures the persistence and expansion of myeloid populations that subvert immune surveillance.
Therapeutic efforts to mitigate the pro-tumoral impact of myeloid cells have intensified, focusing on strategies to disrupt recruitment, survival, and metabolic dependencies. Clinical trials investigating CSF-1R inhibitors aim to curtail TAM infiltration, while CCR2 and CXCR2 blockade targets monocyte and MDSC chemotaxis. Metabolic interventions targeting rate-limiting enzymes, such as 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase 3 (PFKFB3) in glycolysis and carnitine palmitoyltransferase 1A (CPT1A) in FAO, hold promise in reprogramming myeloid phenotypes toward less suppressive states. Concurrent inhibition of arginase and IDO attempts to preserve amino acid availability for effector lymphocytes. However, the clinical translation of such approaches is challenged by compensatory immune mechanisms including polymorphonuclear MDSC infiltration and Treg expansion, highlighting the need for combinatorial and precision therapies.
Advancements in single-cell transcriptomics and spatial proteomics have revolutionized the dissection of myeloid heterogeneity within the TME. Distinct subsets—the likes of TREM2-positive TAMs and PD-L1-expressing macrophages—demonstrate divergent functional roles influenced by tumor type, tissue localization, and metabolic milieu. Unexpectedly, PD-L1+ macrophages have been associated with improved patient prognosis in hepatocellular carcinoma, challenging their traditional categorization as solely immunosuppressive. Similarly, lipid-associated macrophage subsets identified in aggressive triple-negative breast cancer have been implicated in resistance to immune checkpoint blockade, underscoring the heterogeneity and adaptability of these cells.
Explorations into the interplay between myeloid cells and other immune and stromal constituents reveal complex ecosystems driving tumor fate. Interactions with cancer-associated fibroblasts, neutrophils, and B cells create networks that can either facilitate or restrain tumor progression. The conceptual framework of “onco-spheres” envisions tumors as multipartite ecosystems with intricate crosstalk between local and systemic immunity, suggesting that therapeutic success depends on addressing these multifactorial interactions. Modulation of cholesterol metabolism and epigenetic regulators represents emergent avenues to recalibrate myeloid function, potentially enhancing immune responsiveness.
Beyond conventional therapeutic pipelines, repurposing existing drugs such as statins and glutamine antagonists to target metabolic vulnerabilities in myeloid cells offers an exciting translational prospect. β-glucan-like agents that “train” innate immunity indicate a novel paradigm, wherein myeloid cells are reeducated to mount enhanced anti-tumor responses. Such approaches underscore a shift from mere depletion to functional reprogramming of the myeloid compartment, aiming to restore immune equilibrium within the TME.
Addressing therapeutic resistance will require integrated strategies that simultaneously dismantle myeloid cell-mediated immune evasion and potentiate T cell efficacy. This necessitates an expanded focus on transcriptional and metabolic networks that govern cellular plasticity and intercellular signaling. Employing multi-omics integration alongside functional validation will be pivotal in identifying actionable targets that discriminate between pro- and anti-tumor myeloid subsets. Precision immunomodulation may ultimately transform the therapeutic landscape by turning once-pro-cancer myeloid cells into potent allies against malignancy.
The rapidly evolving understanding of myeloid cell biology in oncology heralds a new era of immunotherapy. By unraveling the complex metabolic and signaling circuits underpinning their diverse phenotypes, researchers can systematically exploit these vulnerabilities to reverse immune suppression, inhibit metastasis, and enhance durable responses. The convergence of experimental and computational methodologies promises to deliver personalized interventions that modulate the TME with unprecedented precision, marking myeloid cells not as mere bystanders but as linchpins in the fight against cancer.
Subject of Research: Not applicable
Article Title: Myeloid cells: key players in tumor microenvironments
News Publication Date: 5-Mar-2025
Web References: http://dx.doi.org/10.1007/s11684-025-1124-8
Image Credits: Qiaomin Hua, Zhixiong Li, Yulan Weng, Yan Wu, Limin Zheng
Keywords: Health and medicine
Tags: cancer immunotherapy developmentchemokines in tumor microenvironmentemergency myelopoiesis in cancerextramedullary hematopoiesis in tumorshypoxia effects on immune cellsmyeloid cell plasticity and differentiationmyeloid cells in tumor microenvironmentmyeloid-derived suppressor cells functionregulatory mechanisms of myeloid cellssystemic mobilization of immune cellstumor-associated macrophages role in cancertumor-secreted factors influence on myeloid cells