Highlights
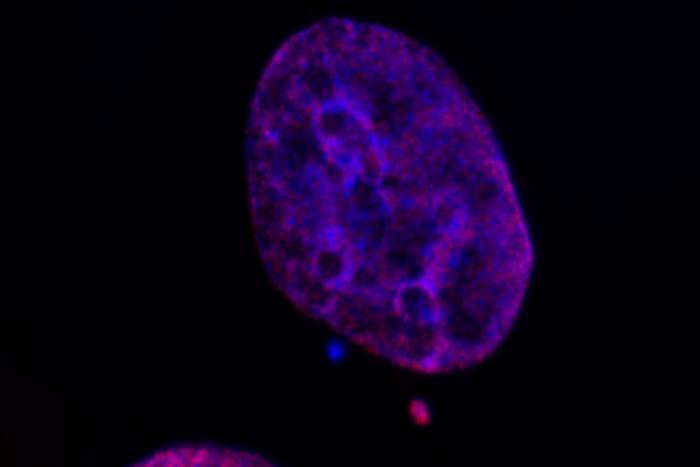
Credit: Image courtesy of the Bakhoum and David Labs, Memorial Sloan Kettering Cancer Center.
Highlights
- MSK-led research found an unexpected link between chromosomal instability and epigenetic alternations, both of which are hallmarks of cancer — especially advanced, drug-resistant cancers.
- Together these defects lead to differences between individual cancer cells within a tumor, a diversity that helps them survive and resist treatment.
- The discovery of this link between the two phenomena points to potential new therapeutic opportunities, as well as opening rich new areas of study.
A graduate student’s curiosity has uncovered a previously unknown link between two important hallmarks of cancer: chromosomal instability and epigenetic alterations.
The resulting study, which was published June 7 in Nature, not only opens a fertile new area of basic science biology research, but has implications for clinical care as well.
Chromosomal instability has to do with changes to the number of chromosomes each cancer cell carries. Epigenetic alterations change which genes get turned on or off in a cell, but without modifying the cell’s DNA code.
In his first year as a doctoral student in pharmacology at Weill Cornell Medicine, Albert Agustinus did a rotation in the lab of Samuel Bakhoum, MD, PhD, whose research group at Memorial Sloan Kettering Cancer Center (MSK) studies how alterations in the number and structure of chromosomes drive cancer. Albert is also co-mentored by epigenetics expert Yael David, PhD, whose lab at MSK’s Sloan Kettering Institute takes a chemical-biology approach to studying the epigenetic regulation of transcription.
“He came to me and said, ‘I’m interested in understanding the link between chromosomal instability and epigenetic modifications,’” Dr. Bakhoum recalls. “And my response to him was, ‘Well, there isn’t a known link, but you’re welcome to find it!’”
And find one he did, expanding that initial inquiry into a 32-author, multi-institution collaboration published in one of science’s top journals. The study was jointly overseen by Bakhoum and David.
Recently, Agustinus recounted his first big “aha” moment in the project, for which he also received a prestigious drug discovery fellowship from the PhRMA Foundation.
He was sitting next to a lab mate and peering through the microscope. The cells he was looking at had abnormal little mini-nuclei scattered throughout the cell — a common consequence of chromosomal instability. And they had been set up with fluorescent markers that would show the presence of epigenetic modifications.
“The micronuclei were glowing much brighter than the primary nucleus,” Agustinus says. “My lab mate said to me, ‘I’ve never seen you smile that wide before.’”
Chromosomes Gone Wild
Chromosomes are tightly packaged strands of DNA that carry our genetic information. Normally, each of our cells has 46 chromosomes — half from one parent and half from the other. When a cell divides to make a new copy of itself, all those chromosomes are supposed to end up in the new cell, but in cancer the process can go dreadfully awry.
“The big question that my lab is trying to answer is how chromosomal instability drives cancer evolution, progression, metastasis, and drug resistance,” Dr. Bakhoum says. “It’s a feature of cancer, especially advanced cancers, where the normal process of cell division goes haywire. Instead of 46 chromosomes, you can have a cell with 69 chromosomes right next to a cell with 80 chromosomes.”
The prevailing wisdom in the field has been that cancer cells increase their chance of survival by shuffling up their genetic material when they divide. This process increases the odds that some of the random changes will allow a cancer daughter cell to withstand the assaults of the immune system and medical interventions.
“This new research, however, suggests that’s only part of the story,” Bakhoum says.
That’s because you can have two cancer cells, each with the same number of extra copies of a given chromosome, but each have different genes that are turned off or on. This is due to additional epigenetic changes.
“Our work further suggests that you don’t actually need mutations in the genes that encode epigenetic-modifying enzymes for epigenetic abnormalities to happen. All you need is to have the ongoing chromosomal instability,” Dr. Bakhoum says. “It’s an unexpected finding, but really important. And it also explains why we often find chromosomal instability and epigenetic abnormalities in advanced, drug-resistant cancers, even when there is no evidence of the types of mutations that we would expect to create epigenetic havoc.”
There and Back Again — Or, What Micronuclei Have to Do With Cancer
Small, extra nuclei in cells — known as micronuclei — like the ones Agustinus saw through the microscope are usually rare and quickly get eliminated by the cell’s natural repair mechanisms. When you get a bunch of them, it’s a signal that something has gone horribly wrong, as happens in cancer.
Like a cell’s primary nucleus, these micronuclei contain packages of genetic material. And when these micronuclei burst — which they frequently do — it causes even more problems, the research team found.
Dr. Bakhoum uses the metaphor of a traveler who picks up a foreign accent and brings it back home. The research demonstrated that the sequestration of chromosomes into micronuclei disrupts the organization of chromatin — a complex of genetic components that get packaged into chromosomes during cell division.
This leads to ongoing epigenetic dysregulation, which continues long after a micronucleus is reintegrated into a cell’s primary nucleus.
And the repeated formation and reincorporation of micronuclei over many cycles of cell division leads to the buildup of epigenetic changes. These, in turn, lead to greater and greater differences between individual cancer cells.
The more variation between individual cancer cells within the same tumor, the more likely it is that some of the cells will be resistant to whatever treatment is being thrown at them — allowing them to survive and continue their runaway growth.
Analyzing Epigenetic Changes
To understand and quantify the epigenetic changes happening inside the cells, the researchers use a series of sophisticated experiments to isolate the micronuclei and examine changes occurring in them compared to the cells’ primary nuclei. This allowed them to see patterns of histone modification — changes to the spools around which DNA winds, which, in turn, change access to the underlying genes.
“This allowed us to ask some important questions, like do we actually get transcription of genes that are important in specific pathways?” Dr. David says. “And the answer is ‘yes.’”
They also compared intact versus ruptured micronuclei — revealing an even greater level of changes in the ones that had burst open.
“We also found there were a lot more accessible promoter regions in the micronuclei than in the primary nuclei,” she adds — promoter regions being DNA sequences near the beginning of a gene that help to initiate transcription, a critical step in gene expression.
In one key experiment, the researchers forced a chromosome to go out into a micronucleus and then allowed it to get reintegrated into the primary nucleus. They compared this adventuresome chromosome to one that stayed put.
“Our model chromosome, which happened to be chromosome Y, showed substantial changes in its epigenetic landscape and accessibility of its DNA,” Dr. David says. “This has major implications because of the significant impact the journey of a chromosome into a micronucleus have on the epigenetic changes of the primary nucleus, which we know play a role in tumor progression and evolution.”
The work, she adds, opens whole new avenues of study.
“Now that we’ve demonstrated that chromosomal instability and epigenetic changes are closely linked, we can go deeper and ask questions about precisely how and why,” Dr. David says.
Findings by another research team from Harvard University and the Dana-Farber Cancer Institute, and published in Nature at the same time found additional evidence that supports the MSK team’s discoveries.
Clinical Implications
More than just shedding light on the changes happening inside cancer cells, the research holds promise for treating patients, as well, the researchers note.
Epigenetic changes are a reversible form of gene regulation — and several classes of drugs have already been developed to work on them.
So, to begin with, chromosomal instability and the presence of micronuclei might be used as a biomarkers to help identify which patients might be more likely to be helped by epigenetic modifying drugs, Dr. Bakhoum says.
Additionally, the findings may pave the way for new therapeutic approaches.
“There’s a question of whether we should be treating chromosomally unstable cells with these epigenetic modifying therapies,” he says. “This research demonstrates epigenetic changes can occur without those mutations being present.”
Moreover, the study also suggests that ongoing research into drugs to target chromosomal instability directly might benefit from being combined with efforts to suppress epigenetic alternations, Dr. Bakhoum adds.
Longer term, another potential avenue would be to explore ways of targeting the micronuclei to preventing them from rupturing, which the research showed was a big driver of epigenetic changes, Dr. David notes.
“I think this is a great example of a fundamental, basic science research discovery that, over the next five years, will open multiple interesting avenues for exploration and potential translation to the clinical setting,” she says.
Agustinus, whose curiosity kicked off the entire project and who led the research effort, sums it up this way, “Chromosomal instability and epigenetic alterations help cancer achieve a population diversity that gives them a better chance to survive and develop. But armed with a new understanding of the relationship between these two phenomena, we should be better able to target them therapeutically.”
Additional Authors, Funding and Disclosures
Additional authors include: Duaa Al-Rawi, Melody Di Bona, Mercedes Duran, Richard P. Koche, John Maciejowski, Andrew McPherson, Danguole Norkunaite, Sohrab P. Shah, Seongmin Choi, Eléonore Toufektchan, Britta Weigelt of MSK. Vivek Mittal and Shira Yomtoubian of Weill Cornell Medicine. Vineet Bafna, Bhargavi Dameracharla, and Jens Luebeck of the University of California, San Diego. Robert M. Myers of the New York Genome Center and Tri-Institutional MD-PhD Program; Dan Landau of the New York Genome Center and Weill Cornell Medicine; and Ramya Raviram of the New York Genome Center. Mathieu F. Bakhoum and Bailey S. C. L. Jonesof the Yale School of Medicine. Simone Sidoli and Stephanie Stransky of the Albert Einstein College of Medicine. Enrico Gratton and Lorenzo Scipioni of the University of California, Irvine. Kristina Keuper and Zuzana Storchova of the University of Kaiserslautern, Germany. Paul S. Mischel of Stanford University. And Peter Ly of the University of Texas Southwestern Medical Center.
The work was supported by the National Institutes of Health (DP5OD026395, P50CA192937, R35GM138386, 1S10OD030286-01, T32-CA009207, U24CA264379, R01GM114362, T32GM007739, P30 CA016359, P30-CA008748), National Cancer Institute (P50CA247749, R01CA256188-01, R00CA212290, R37CA261183, R01CA270102), Congressionally Directed Medical Research Program, Burroughs Wellcome Fund, Josie Robertson Foundation, Mark Foundation for Cancer Research, Pershing Square Sohn Cancer Research Alliance, Parker Institute for Cancer Immunotherapy, Anna Fuller Trust, Leukemia Research Foundation, Hollis Brownstein New Investigator Research Grant, American Federation for Aging Research; Deerfield, Relay Therapeutics, Merck, PhRMA Foundation, Mary Jane Milton Endowed Fellowship in Gynecologic Oncology and Connecticut Lions Eye Research Foundation.
MSK Disclosures: Samuel Bakhoum owns equity in, receives compensation from, and serves as a consultant and the scientific advisory board and board of directors of Volastra Therapeutics. Richard P. Koche is a co-founder of and consultant for Econic Biosciences. Sohrab Shah is a shareholder in Imagia Canexia Health and is a consultant with Astra Zeneca, outside the study. Britta Weigelt reports a research grant from Repare Therapeutics, outside the study.
Journal
Nature
DOI
10.1038/s41586-023-06084-7
Method of Research
Experimental study
Subject of Research
Cells
Article Title
Epigenetic dysregulation from chromosomal transit in micronuclei
Article Publication Date
7-Jun-2023
COI Statement
MSK Disclosures: Samuel Bakhoum owns equity in, receives compensation from, and serves as a consultant and the scientific advisory board and board of directors of Volastra Therapeutics. Richard P. Koche is a co-founder of and consultant for Econic Biosciences. Sohrab Shah is a shareholder in Imagia Canexia Health and is a consultant with Astra Zeneca, outside the study. Britta Weigelt reports a research grant from Repare Therapeutics, outside the study.



