Researchers at Memorial Sloan Kettering Cancer Center (MSKCC) in New York have discovered that common cancer treatments, such as radiotherapy or anthracycline drugs, cause long-term damage to heart tissue by activating a key inflammatory signaling pathway. The study, published December 19 in the Journal of Experimental Medicine (JEM), suggests that inhibiting this pathway could reduce the chances of cancer survivors suffering heart disease later in life.
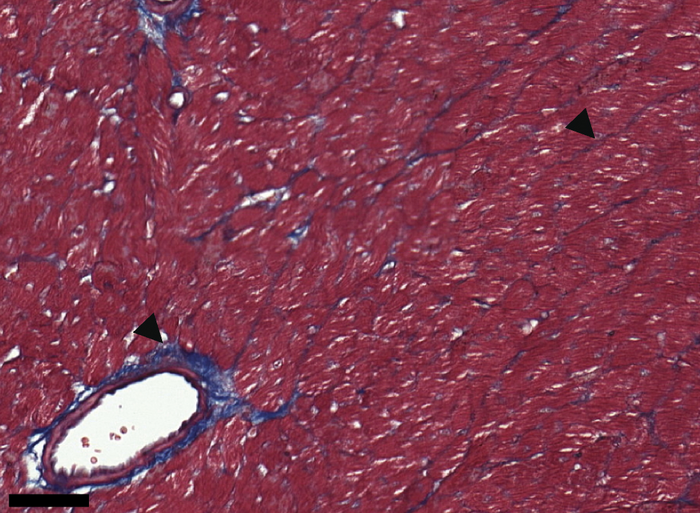
Credit: © 2022 Shamseddine et al. Originally published in Journal of Experimental Medicine. https://doi.org/10.1084/jem.20220809
Researchers at Memorial Sloan Kettering Cancer Center (MSKCC) in New York have discovered that common cancer treatments, such as radiotherapy or anthracycline drugs, cause long-term damage to heart tissue by activating a key inflammatory signaling pathway. The study, published December 19 in the Journal of Experimental Medicine (JEM), suggests that inhibiting this pathway could reduce the chances of cancer survivors suffering heart disease later in life.
Many cancers are treated with radiation and/or drugs that kill tumor cells by causing breaks in their DNA. But these treatments also damage the DNA of the patient’s healthy cells. As the survival rates of cancer patients continue to rise, the long-term consequences of this are an increasing area of concern. For example, radiotherapy or a class of DNA-damaging drugs known as anthracyclines can have delayed, toxic effects on the heart, increasing the risk of developing cardiovascular diseases, including coronary artery disease or heart failure. One study found that the occurrence of cardiovascular disease is five times higher in long-term survivors of Hodgkin’s lymphoma than it is in the general population.
“The mechanisms by which DNA damage leads to late tissue toxicity years after cancer treatment have been poorly understood,” says Adam M. Schmitt, a radiation oncologist at MSKCC. “Identifying the pathogenic mechanisms of toxicity and early biomarkers of their activation would provide an opportunity to intervene with treatment to prevent toxicity.”
In their study, Schmitt and colleagues found that one month after mice were exposed to radiation or anthracyclines, a specific population of heart cells called fibroblasts activated a set of genes that promoted the recruitment of various immune cell types associated with pathological inflammation and tissue fibrosis. Within 3–6 months, the mice developed signs of cardiac dysfunction, and, by 12 months, many of them had died of heart failure.
The researchers determined that this pathological process is driven by an immune signaling pathway called the cGAS–STING pathway. This pathway usually promotes inflammation in response to DNA fragments derived from pathogenic bacteria or viruses, but, Schmitt and colleagues reasoned, it could also be activated by DNA fragments generated in response to radiation or anthracycline treatment.
Mice lacking either the cGAS or STING proteins were protected from the toxic side effects of DNA-damaging cancer treatments. They showed no signs of cardiac inflammation, maintained normal heart function, and were still alive a year after treatment. A small molecule inhibitor of the STING protein also protected mice from the toxic effects of radiotherapy or anthracyclines.
By studying breast cancer patients treated with anthracyclines, Schmitt and colleagues found evidence that the cGAS–STING pathway may play a similarly important role in human cardiac toxicity. One of the key inflammatory proteins induced by cGAS–STING signaling is CXCL10, and the researchers found that patients who showed the largest increases in CXCL10 levels after anthracycline treatment subsequently showed changes on echocardiograms associated with cardiac toxicity.
“Taken together, our data reveal that targeting of the cGAS–STING pathway holds great potential as a treatment to prevent cardiac complications of DNA-damaging cancer treatments,” Schmitt says. “These data indicate that clinical trials utilizing cGAS–STING inhibitors to prevent cardiovascular disease following DNA-damaging cancer therapy are warranted.”
Shamseddine et al. 2022. J. Exp. Med. https://rupress.org/jem/article-lookup/doi/10.1084/jem.20220809?PR
# # #
About Journal of Experimental Medicine
Journal of Experimental Medicine (JEM) publishes peer-reviewed research on immunology, cancer biology, stem cell biology, microbial pathogenesis, vascular biology, and neurobiology. All editorial decisions on research manuscripts are made through collaborative consultation between professional scientific editors and the academic editorial board. Established in 1896, JEM is published by Rockefeller University Press, a department of The Rockefeller University in New York. For more information, visit jem.org.
Visit our Newsroom, and sign up for a weekly preview of articles to be published. Embargoed media alerts are for journalists only.
Follow JEM on Twitter at @JExpMed and @RockUPress.
Journal
Journal of Experimental Medicine
DOI
10.1084/jem.20220809
Method of Research
Experimental study
Subject of Research
Animals
Article Title
Innate immune signaling drives late cardiac toxicity following DNA-damaging cancer therapies
Article Publication Date
19-Dec-2022
COI Statement
Disclosures: J.E. Liu reported consulting for Pfizer and Caption
Health, honoraria from Philips, and Data and Safety Monitoring
Board for Caelum Biosciences. N.D. Socci reported grants from
National Institutes of Health during the conduct of the study.
S.F. Bakhoumreported personal fees fromVolastra Therapeutics
Inc. and Sanofi and other support from Cancer Research UK and
Prostate Cancer Foundation outside the submitted work; in addition,
S.F. Bakhoum has a patent for targeting chromosomal
instability and downstream cytosolic DNA signaling for cancer
treatment licensed to Volastra Therapeutics Inc. and a patent for
methods and strategies to target cytosolic dsDNA signaling in
chromosomally unstable cancers pending. A.M. Schmitt reported
spouse is an employee of Regeneron Pharmaceuticals. No
other disclosures were reported.




