In a groundbreaking collaborative effort, researchers at The University of Texas MD Anderson Cancer Center, in partnership with biotechnology firm BostonGene, have achieved a significant milestone in understanding and targeting renal medullary carcinoma (RMC), one of the most aggressive and rare forms of kidney cancer. This malignancy predominantly affects young individuals, particularly those with sickle cell trait, and has long been characterized by its rapid progression and poor response to conventional therapies. The team’s comprehensive molecular analysis of RMC tumor samples, involving the largest cohort to date, has unveiled critical insights into the cancer’s biology, leading to the identification of TROP2 as a pivotal therapeutic target.
Renal medullary carcinoma remains an intractable challenge for oncologists due to its aggressive nature and lack of effective treatments. This tumor arises in the kidney’s medullary region and is notoriously resistant to chemotherapy and radiation, resulting in dismal patient outcomes. Understanding the molecular underpinnings of RMC has been limited by the rarity of the disease and the scarcity of well-characterized tumor samples. Overcoming this barrier, the MD Anderson and BostonGene team meticulously analyzed 25 patient-derived RMC specimens using state-of-the-art genomic, proteomic, and transcriptomic technologies, providing an unprecedented molecular portrait of this lethal cancer type.
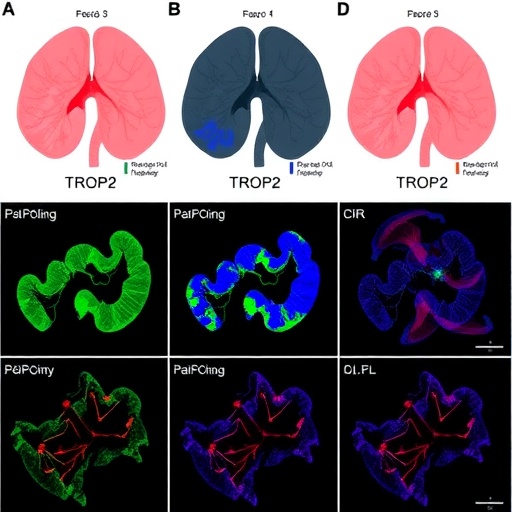
The focal point of their investigation was the identification of cell surface proteins that are aberrantly expressed in RMC tumors, as such molecules represent accessible targets for antibody-based therapies. Among these, TROP2 — a transmembrane glycoprotein involved in cell proliferation and survival signaling pathways — emerged as robustly overexpressed across the tumor samples. This finding was particularly significant as TROP2 had previously been implicated in other epithelial malignancies but was unexplored in the context of RMC. The overexpression of TROP2 in RMC suggests a potential vulnerability that can be exploited therapeutically.
In parallel, the research delineated the activity of intracellular signaling cascades within RMC tumors, notably highlighting increased activation of the Hippo signaling pathway. This pathway plays a fundamental role in regulating organ size, cell proliferation, and apoptosis, and its dysregulation is increasingly recognized in various cancers. The upregulation of Hippo signaling correlates with the aggressive phenotype of RMC, further underscoring the complex molecular environment sustaining tumor growth and resistance.
Building on these molecular insights, the researchers investigated the therapeutic potential of sacituzumab govitecan, an antibody-drug conjugate that selectively targets TROP2-expressing cells. This agent combines a monoclonal antibody directed at TROP2 with a potent chemotherapeutic payload, delivering cytotoxic agents directly to cancer cells while sparing normal tissue. The precision of this approach represents an evolution beyond traditional chemotherapy and offers hope in a cancer type traditionally refractory to treatment.
In a compassionate-use clinical evaluation involving four heavily pretreated RMC patients, sacituzumab govitecan demonstrated promising preliminary activity. Among these patients, there was one documented partial response, marked by a significant reduction in tumor burden, and two additional patients experienced stable disease, indicating disease control for a period. The median progression-free survival in this small cohort was recorded at 2.9 months, a noteworthy endpoint given the historical progression metrics of RMC patients.
While these clinical outcomes highlight the potential impact of TROP2-targeted therapy, they also reflect the inherent challenges posed by RMC’s aggressiveness and heterogeneity. The disease’s rapid progression necessitates early intervention, and the variability in patient responses underscores the need for further optimization and combination approaches. Nonetheless, the demonstration of clinical benefit, even in a small cohort, provides a compelling rationale for larger, more definitive trials to validate sacituzumab govitecan’s efficacy in this context.
One of the study’s profound contributions lies in its elucidation of the RMC tumor microenvironment, an often-overlooked aspect influencing tumor growth and therapeutic resistance. The microenvironment comprises not just cancer cells but also immune cells, stromal components, and vascular structures, all of which interact dynamically to modulate tumor behavior. Understanding these interactions may reveal additional therapeutic targets and inform combination strategies that enhance the efficacy of TROP2-directed therapies.
The identification of TROP2 as a therapeutic target in RMC signals a paradigm shift towards precision oncology for this rare cancer. Precision medicine aims to tailor treatments based on the unique molecular characteristics of a patient’s tumor, maximizing efficacy while minimizing toxicity. For a malignancy with limited treatment options and poor prognosis, this approach offers a critical pathway for improving survival and quality of life.
Furthermore, this study emphasizes the importance of collaborative research frameworks that bridge academic institutions and biotechnology companies. The alliance between MD Anderson and BostonGene combined clinical expertise with cutting-edge molecular diagnostics and drug development capabilities, illustrating the power of multidisciplinary partnerships in accelerating translational cancer research.
The research received partial funding support from the National Cancer Institute, signifying the strategic prioritization of investigating rare but deadly cancers like RMC within the broader cancer research agenda. Such investments are essential to foster innovation and generate breakthroughs that might otherwise remain elusive due to the small patient populations affected.
Looking ahead, the findings from this extensive molecular characterization serve as a foundation for expanding the therapeutic arsenal against RMC. They encourage exploration of other cell surface antigens and signaling pathways contributing to the malignant phenotype. Moreover, integrating TROP2-targeted agents with immune checkpoint inhibitors or other novel therapeutic modalities could potentiate treatment responses.
In conclusion, the pioneering work by MD Anderson and BostonGene researchers marks a pivotal advancement in the fight against renal medullary carcinoma. By leveraging molecular insights to identify TROP2 as a viable target and demonstrating preliminary clinical efficacy of sacituzumab govitecan, they have opened new avenues for therapeutic intervention in a cancer that has eluded effective treatment. This breakthrough stands not only as a beacon of hope for patients afflicted with RMC but also exemplifies the transformative potential of precision oncology approaches applied to rare and difficult-to-treat cancers.
Subject of Research: Renal Medullary Carcinoma (RMC) and Therapeutic Targeting of TROP2
Article Title: Identification of TROP2 as a Therapeutic Target in Renal Medullary Carcinoma
News Publication Date: 30-Oct-2025
Web References:
Full paper: https://www.cell.com/cell-reports-medicine/fulltext/S2666-3791(25)00496-3
DOI: http://dx.doi.org/10.1016/j.xcrm.2025.102423
Keywords: Renal Medullary Carcinoma, Kidney Cancer, TROP2, Hippo Pathway, Sacituzumab Govitecan, Antibody-Drug Conjugate, Precision Oncology, Tumor Microenvironment, Molecular Characterization
Tags: aggressive kidney cancer treatmentcollaborative cancer research effortsgenomic and proteomic technologieskidney cancer prognosismolecular profiling in canceroncological challenges in RMCpatient-derived tumor analysisrare cancer molecular insightsrenal medullary carcinoma researchsickle cell trait and cancertargeted therapies for RMCTROP2 therapeutic target