In a groundbreaking study that could reshape therapeutic strategies for vascular diseases, researchers have unveiled a pivotal mechanism behind angiogenesis—the formation of new blood vessels—through the transfer of mitochondria from adipose-derived regenerative cells (ADRCs). This fascinating discovery sheds light on the cellular interactions that facilitate healing in ischemic conditions, particularly in a murine hindlimb ischemia model, indicating potential pathways for advanced regenerative medicine.
The research carried out by a team led by Che, Shimizu, and Hayashi delves deep into the challenges presented by ischemic conditions, like peripheral artery disease. These conditions often lead to severe complications, including limb loss, which heightens the urgency for novel therapeutic approaches. Utilizing innovative methodologies, the authors aim to illustrate how ADRCs can facilitate angiogenesis, offering hope for improved outcomes in ischemic patients.
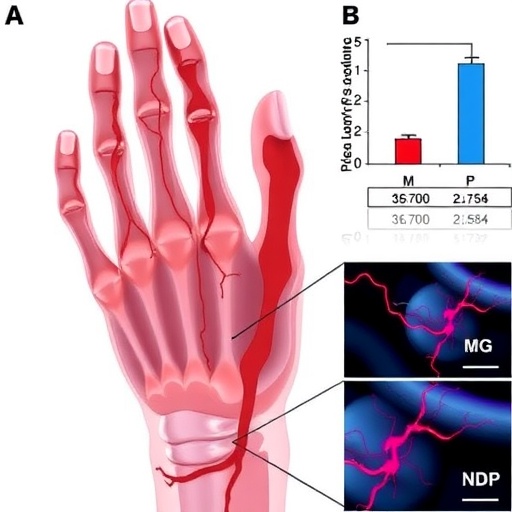
At the heart of their findings is the concept of mitochondrial transfer—a process where mitochondria from one cell are transferred to another. This communication between cells is critical, as mitochondria are known as the powerhouse of the cell, generating the energy necessary for cellular functions. The researchers investigated whether the transfer of these organelles from ADRCs could bolster the regenerative capacity of damaged tissues, particularly in the context of ischemic hindlimb conditions.
In their study, the researchers employed a murine model that closely simulates human ischemic conditions. By subjecting these mice to hindlimb ischemia, they mimicked the blood flow deprivation caused by arterial blockages. In this controlled setting, the role of ADRCs became focal, especially regarding their ability to transfer mitochondria to injured cells in the affected limbs. This cellular exchange was not only unprecedented but also presented a significant advancement in our understanding of tissue repair mechanisms.
The impressive ability of ADRCs to not only survive but thrive under adverse conditions was emphasized. These stem cell-like entities exhibit unique properties that allow them to migrate towards sites of injury, creating a conducive environment for healing. By enabling mitochondrial transfer, these cells may enhance the metabolic function of compromised tissues, offering a dual approach to tissue recovery—by providing new cellular energy and potentially rejuvenating existing damaged cells.
The implications of this research extend beyond mere cellular biology. The findings suggest that harnessing the power of ADRCs could lead to innovative therapies aimed at restoring blood flow and restoring tissue viability in ischemic patients. By applying this regenerative approach, clinicians may alleviate the debilitating effects of vascular diseases and improve the quality of life for countless individuals affected by such conditions.
Furthermore, the researchers meticulously explored the signaling pathways involved in the mitochondrial transfer process. They uncovered the key factors influencing this transfer, shedding light on how ADRCs communicate with recipient cells. The identification of these molecular players could pave the way for the development of targeted therapies that enhance mitochondrial transfer in clinical settings.
As the research community continues to examine the broader implications of this study, discussions around the ethical considerations of cell-based therapies will undoubtedly arise. The potential for adipose-derived regenerative cells to undergo large-scale clinical applications hinges not only on their efficacy but also on the ethical frameworks that guide their use. Thorough investigations into how these therapies can be safely integrated into existing medical practices are crucial as this field of regenerative medicine advances.
Moreover, the collaboration among the researchers exemplifies the growing trend of interdisciplinary approaches in science. By combining expertise from various domains, including cell biology, regenerative medicine, and medical ethics, they have laid a foundation for future innovations. The cross-pollination of ideas and techniques from multiple fields can catalyze breakthroughs that push the boundaries of what is currently conceivable in regenerative therapies.
Despite the promising results, the researchers acknowledge the need for further exploration. Future studies will need to assess the long-term outcomes of mitochondrial transfer in larger clinical models to confirm the efficacy and safety of ADRCs in angiogenesis. Addressing potential challenges, such as immune response or varying efficacy across different patient populations, will also be essential before these findings can be translated into routine clinical use.
In conclusion, the research team’s innovative work on mitochondrial transfer from adipose-derived regenerative cells sets the stage for a new era in regenerative medicine. By elucidating the mechanisms of angiogenesis in ischemic conditions, they have opened new avenues for therapeutic interventions that could significantly alter the landscape of treatment for vascular diseases. As this research progresses, continued exploration will be essential to fully realize its potential in clinical settings, providing hope for improved outcomes in patients suffering from ischemia and related conditions.
The journey from bench to bedside in regenerative therapies promises not only to enhance patient care but also to inspire further research aimed at understanding the intricate ballet of cellular communication in the healing process. With continued investment in this field, the vision of leveraging adipose-derived regenerative cells to combat ischemic diseases may soon be a reality, changing lives and restoring functionality for many.
Subject of Research: Mitochondrial transfer from adipose-derived regenerative cells and its role in therapeutic angiogenesis.
Article Title: Mitochondrial transfer from adipose-derived regenerative cells contributes therapeutic angiogenesis in a murine hindlimb ischemia model.
Article References:
Che, Y., Shimizu, Y., Hayashi, T. et al. Mitochondrial transfer from adipose-derived regenerative cells contributes therapeutic angiogenesis in a murine hindlimb ischemia model. Angiogenesis 28, 49 (2025). https://doi.org/10.1007/s10456-025-10001-z
Image Credits: AI Generated
DOI: https://doi.org/10.1007/s10456-025-10001-z
Keywords: mitochondrial transfer, adipose-derived regenerative cells, therapeutic angiogenesis, ischemia, regenerative medicine, murine model, vascular diseases, cell therapy.
Tags: adipose-derived regenerative cellsadvances in vascular regenerative medicineangiogenesis in ischemic conditionscellular communication in healingenhancing healing through cell therapymechanisms of blood vessel formationmitochondrial function in tissue repairmitochondrial transfer in regenerative medicinemurine hindlimb ischemia modelnovel therapies for vascular diseasesperipheral artery disease treatmentregenerative strategies for limb ischemia