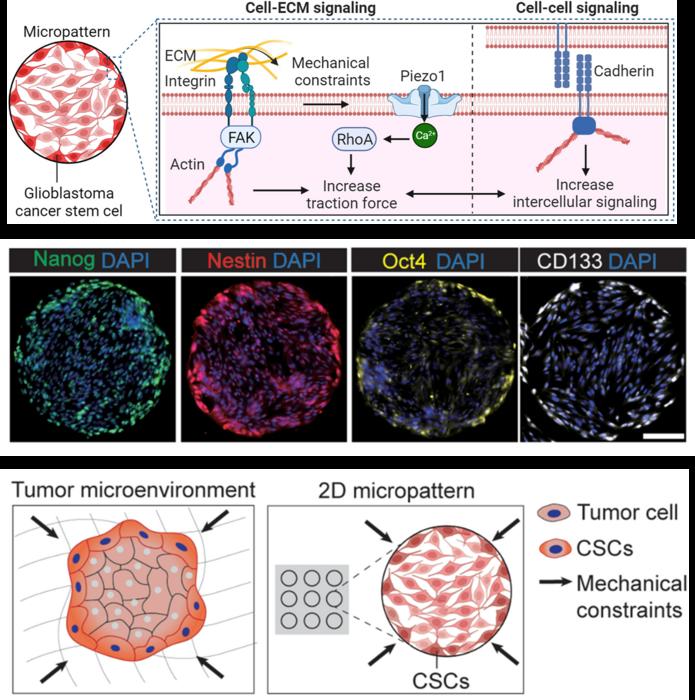
A new study led by Prof. Chen from the Department of Biomedical Engineering at New York University discovered the function of mechanical constraints in glioblastoma. This study explored the underlying mechanotransductive machinery involved in the mechanical constraints-mediated emergence and spatial patterning of CSCs in GBM. Using a 2D micropatterned multicellular model, They revealed that Piezo1, collaborating with focal adhesions, cadherins, and downstream cytoskeletal machinery, regulates mechanosensing of cancer cells to mechanical constraints in the GBM tumor microenvironment and guide the spatial patterning of in CSCs.
Credit: Ngoc Luu, Shuhao Zhang, Raymond H.W. Lam, and Weiqiang Chen
A new study led by Prof. Chen from the Department of Biomedical Engineering at New York University discovered the function of mechanical constraints in glioblastoma. This study explored the underlying mechanotransductive machinery involved in the mechanical constraints-mediated emergence and spatial patterning of CSCs in GBM. Using a 2D micropatterned multicellular model, They revealed that Piezo1, collaborating with focal adhesions, cadherins, and downstream cytoskeletal machinery, regulates mechanosensing of cancer cells to mechanical constraints in the GBM tumor microenvironment and guide the spatial patterning of in CSCs.
Glioblastoma (GBM) is the most aggressive type of brain tumor originating from glial cells. It is characterized by a high fraction of cancer cells with stem-like properties in their tumor microenvironment. This subpopulation of cancer cells, referred to as “cancer stem cells” (CSCs), are defined by their self-renewal and tumor-initiating capacities. CSCs are tightly associated with tumorigenesis, metastasis, high resistance to cancer treatments, and poor survival rates in patients. Despite their prognosis significance, the origin of CSCs in GBM is not yet fully understood. The emergence of CSCs could originate from both intrinsic and extrinsic factors.
A major mechanical characteristic of GBM is the accumulated stress inside the tumor due to the physical confinement of the tumor by the rigid skull. In this scenario, GBM cells in the overcrowding tumor microenvironment undergo mechanical constraints that impact both the cell body and nucleus, leading to numerous cellular alterations such as cell deformability, traction force and cancer cell DNA transcription. In addition, gradients of mechanical stresses generated within the confined tumor microenvironment can mechanically induce a malignant phenotype in cancer cells at different regions and influence the emergence and spatial distribution of CSCs within the tumor. Recent studies have found that the distribution of CSCs in tumor tissue is not uniform both in vitro and in vivo. For instance, the EMT and CSCs preferentially occur in the peripheral regions of cell monolayers. Although plenty of evidence supports that these mechanical cues in the tumor microenvironment influence CSC phenotype and mechanics, it remains unclear how cooperative intracellular and intercellular forces due to mechanical constraints in tumors regulate the emergence and spatial patterning of CSCs in GBM. More importantly, little is known about the underlying mechanosensitive signaling that initiates CSC emergence and maintains the spatial heterogeneity of CSC in GBM tumor.
In this study, Chen’s Lab uses a two-dimensional (2D) micropatterned multicellular model to examine the impact of mechanical constraints in tumor on the emergence and spatial patterning of CSCs in GBM. They specifically investigated how the interplay between forces arising from the cell-ECM and cell–cell interactions guide the emergence and spatial distribution of CSCs in GBM. In different geometric multicellular patterns, GBM cells in the peripheral regions are all found to express a higher level of CSC makers. The distinct spatial pattern of CSCs was found to correspond to the gradients of mechanical stresses generated within the confined environment. They further highlighted the coordination among Piezo1 mechanosensitive channels, integrins, and cadherins in regulating GBM cell mechanosensing and demonstrated that the upregulation of Piezo1 plays a critical role in the phenotypic switch of GBM cells to CSCs. These findings highlight the role of mechanical forces that originate from cellular contractility and intercellular interaction, which emerged from the mechanical constraints in the multicellular organization, in determining the unique spatial patterns of CSCs in GBM.
Journal
Mechanobiology in Medicine
DOI
10.1016/j.mbm.2023.100027
Method of Research
Experimental study
Subject of Research
Cells
Article Title
Mechanical constraints in tumor guide emergent spatial patterns of glioblastoma cancer stem cells
Article Publication Date
1-Dec-2023



