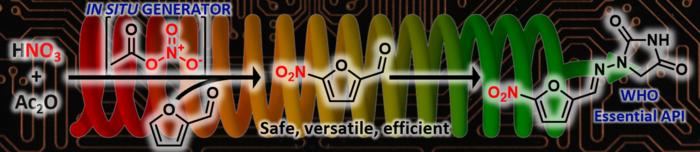
In a groundbreaking advancement that promises to reshape pharmaceutical manufacturing, researchers at the University of Liège in Belgium have developed an innovative automated continuous flow system tailored to produce key antibacterial drugs from bio-based furfural more safely and efficiently. This pioneering technology, a result of international collaboration supported by the U.S. Food and Drug Administration (FDA), offers a transformative approach to the well-known challenges of nitration chemistry, particularly when working with sensitive biomass-derived molecules. Published open access in Angewandte Chemie International Edition, the study introduces a new era in sustainable drug synthesis through advanced automation and continuous flow chemistry.
Nitration reactions have historically been among the most perilous chemical transformations due to the inherent instability and explosive potential of nitrating agents. Conventional nitration methodologies suffer from significant drawbacks, including the use of harsh reagents, poor selectivity, and safety hazards, all of which are amplified when handling delicate bio-based substrates such as furfural. Furfural is recognized as a valuable platform chemical derived from lignocellulosic biomass, serving as a precursor to nitrofuran-based antibiotics. However, its instability under traditional reaction conditions often results in inconsistent yields and compromised reproducibility, limiting its practical application in pharmaceutical production.
Addressing these critical challenges, scientists at the Center for Integrated Technology and Organic Synthesis (CiTOS) at the University of Liège have engineered an automated continuous flow platform that enables the in situ generation and immediate consumption of acetyl nitrate, a milder and more selective nitrating agent. This strategic innovation circumvents the hazards and inefficiencies traditionally associated with nitration by integrating real-time monitoring and precise reaction parameter control within a modular flow design. As a result, the system achieves superior safety profiles, scalability, and reproducibility in the synthesis of nitrofuran drug precursors.
Professor Jean-Christophe Monbaliu, Director of CiTOS, emphasizes the unique marriage of cutting-edge automation with practical simplicity that characterizes this system. According to him, the platform’s capacity for remote operation by a single technician represents a quantum leap forward in pharmaceutical manufacturing safety and efficiency. The versatility of the design also allows for the production of multiple nitrofuran antibiotics within the same hardware setup, underscoring its adaptability and potential impact on industrial drug synthesis pipelines.
The crux of the innovation lies in the controlled generation of acetyl nitrate within a sequence of interconnected reactor modules. This continuous flow configuration harnesses advanced analytical technologies—including inline infrared (IR) and ultraviolet-visible (UV-Vis) spectroscopy—to provide real-time insight into reaction progress. The reactors are equipped with precise temperature and pressure sensors, ensuring optimal control throughout the process. An integrated automated separation unit further enhances product purity by efficiently isolating the desired nitrated compounds immediately upon synthesis.
One of the most significant hurdles in nitration chemistry is the handling and storage of acetyl nitrate, an inherently unstable and hazardous reagent. The CiTOS team skillfully sidesteps these risks by synthesizing acetyl nitrate on demand within the flow system and consuming it instantaneously. This design virtually eliminates the accumulation of dangerous intermediates, substantially reducing the potential for accidents while maintaining tight control over reaction kinetics and selectivity.
The efficacy of this continuous flow platform was demonstrated through rapid synthesis of four antibacterial compounds recognized by the World Health Organization for their clinical significance. Remarkably, each compound was produced in under five minutes, highlighting the system’s exceptional throughput capabilities. The resulting products exhibited outstanding purity and high yields, validating the platform’s practical utility for scalable pharmaceutical manufacturing.
Lead author Hubert Hellwig, a senior postdoctoral researcher at CiTOS, underscores that this development transcends mere chemical innovation. The project incorporated custom-designed modules, sophisticated electronics, and a robust control infrastructure, all orchestrated through an integrated software framework. Importantly, every detail—from hardware blueprints to operational data—has been made freely accessible to promote transparency, reproducibility, and adoption within the global scientific community.
This open-access ethos not only accelerates technology dissemination but also aligns with broader objectives to foster safer and more sustainable approaches in chemical manufacturing. By minimizing hazards associated with nitration and enabling the use of renewable biomass feedstocks like furfural, this platform offers a compelling blueprint for greener pharmaceutical production. The implications extend beyond nitrofuran antibiotics, with potential adaptability to a wide range of nitrated intermediates essential in diverse therapeutic areas.
The success of this initiative was bolstered by a collaborative consortium including partners from the Massachusetts Institute of Technology, Northeastern University, the University of Puerto Rico, and the National Institute for Pharmaceutical Technology. Such interdisciplinary cooperation underscores the global priority placed on improving chemical synthesis safety and sustainability in the pharmaceutical sector.
Looking forward, this automated continuous flow technology could serve as a springboard for developing even more complex multi-step syntheses in a streamlined, fully automated manner. By enabling direct integration of reaction, monitoring, and purification stages within a single continuous system, it promises to revolutionize how active pharmaceutical ingredients are manufactured at scale. This is especially pertinent as the industry seeks resilient, safe, and eco-friendly solutions amid evolving regulatory and environmental pressures.
Subject of Research: Automated continuous flow synthesis for safe and efficient production of nitrofuran pharmaceuticals from bio-based furfural.
Article Title: Continuous Flow Synthesis of Nitrofuran Pharmaceuticals using Acetyl Nitrate
News Publication Date: 12-May-2025
Web References:
DOI: 10.1002/anie.202501660
Image Credits: Hubert Hellwig, CiTOS, University of Liège
Keywords
Continuous flow chemistry, nitration, acetyl nitrate, furfural, nitrofuran antibiotics, automation, pharmaceutical manufacturing, biomass-derived chemicals, green chemistry, process safety, modular reactors, real-time monitoring
Tags: automated continuous flow chemistrybio-based furfural applicationschallenges in nitration reactionscontinuous flow system for pharmaceuticalsFDA-supported pharmaceutical researchinnovative antibacterial drug productionnitration chemistry advancementsreducing hazards in chemical reactionssafer pharmaceutical manufacturingstability of biomass-derived chemicalssustainable drug synthesis technologytransformative approaches in drug synthesis