Scientists gain insights into how pathogenic bacteria of the genus Bartonella give rise to lesions in the human body, opening new avenues in modern medicine
Credit: Kentaro Tsukamoto
Bacteria of the genus Bartonella are parasites that can be transmitted to humans via insect bites and animal scratches, resulting in an infection known as “bartonellosis.” Cat-scratch disease and trench fever are forms of bartonellosis caused by different Bartonella species infecting humans. Bartonella bacteria can cause lesions to pop up in the skin and internal organs. To provide themselves with a safe habitat, the bacteria bring about the increase of the number of “vascular endothelial” cells (cells that line the interior of blood vessels), which hide themselves from the host immune system and stimulate the creation of new blood vessels, through a process called “angiogenesis.”
Previous studies on Bartonella henselae (B. henselae for short), the bacterium responsible for cat-scratch disease, have shown that it can directly “inject” proteins that inhibit programmed cell death (apoptosis) into the endothelial cells. However, B. henselae can also promote angiogenesis without directly contacting endothelial cells, which implies that the bacterium can secrete a bioactive substance that takes on the duty of kick-starting angiogenesis.
In a new study published in Nature Communications, a team of scientists led by Senior Assistant Professor Kentaro Tsukamoto and Professor Yohei Doi of Fujita Health University, Japan, have identified that this bioactive substance is actually a protein. They have also named this protein as Bartonella angiogenic factor A, or “BafA” for short. This is the very first report of a vascular endothelial growth factor (VEGF for short)-like protein produced by bacteria.
The scientists started their project by introducing B. henselae into human endothelial cells in petri dishes, and observed that the bacteria caused the endothelial cells to multiply. To identify the genes that give B. henselae this ability, the researchers began inducing random mutations in the DNA of the bacteria and seeing whether the mutated bacteria could still make the endothelial cells multiply. Through these experiments, the scientists determined that B. henselae can stimulate angiogenesis in human endothelial cells only if it possesses a functional copy of the gene that “codes for,” or guides the synthesis of, the BafA protein. They also observed that exposing human endothelial cells to the isolated BafA protein caused the cells to multiply.
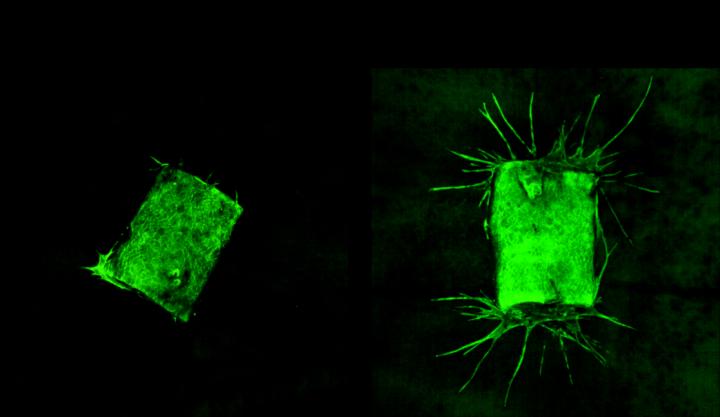
Then, to confirm that BafA stimulates angiogenesis, the scientists extracted samples of a major blood vessel called the aorta from mice and placed the samples in gels that did or did not contain BafA. As can be seen in the image below, the aorta samples that were not exposed to BafA did not sprout new blood vessels, but the aorta samples that were exposed to BafA grew vessels that extended into the gel. The scientists also found that surgically placing a BafA-containing gel plug into living mice led to blood vessels growing from the surrounding tissue into the gel.
Further experiments with human endothelial cells in petri dishes indicated that BafA activated cell surface receptors that recognize VEGF. By binding to these receptors, BafA triggered the activation of a process inside the cells, involving proteins called mitogen-activated protein kinase (MAPK) and extracellular signal-regulated kinases (ERKs). The MAPK/ERK pathway plays an important role in the multiplication of endothelial cells and angiogenesis. “In the last set of experiments, we performed similar studies in a related bacterium called Bartonella quintana, the bacterium that causes trench fever, and we found that it produces its own version of BafA that also causes human endothelial cells to multiply,” explains Dr Tsukamoto.
These findings provide valuable insights into the mechanisms by which infectious bacteria can produce lesions in their hosts. “We believe that BafA proteins can be leveraged as tools for studying angiogenesis, and we also consider potential medical benefits,” reports Prof Doi. “Most importantly,” he elaborates, “BafA is a potential target for the development of diagnostic and therapeutic strategies for bartonellosis.”
The scientists also speculate that BafA proteins could be used in regenerative medicine, which is a highly specialized branch of medicine that deals with replacing or regenerating lost or damaged parts of the body. Further research is needed to confirm the scientists’ findings, but needless to say, BafA proteins will certainly be of immense interest to the scientific community.
###
Reference
Title of original paper: The Bartonella autotransporter BafA activates the host VEGF pathway to drive angiogenesis
Journal: Nature Communications
DOI: 10.1038/s41467-020-17391-2
About Fujita Health University
Fujita Health University is a private university situated in Toyoake, Aichi, Japan. It was founded in 1964 and houses one of the largest teaching university hospitals in Japan in terms of the number of beds. With over 900 faculty members, the university is committed to providing various academic opportunities to students internationally. Fujita Health University has been ranked eighth among all universities and second among all private universities in Japan in the 2020 Times Higher Education (THE) World University Rankings. THE University Impact Rankings 2019 visualized university initiatives for sustainable development goals (SDGs). For the “good health and well-being” SDG, Fujita Health University was ranked second among all universities and number one among private universities in Japan. The university will also be the first Japanese university to host the “THE Asia Universities Summit” in June 2021. The university’s founding philosophy is “Our creativity for the people (DOKUSOU-ICHIRI),” which reflects the belief that, as with the university’s alumni and alumnae, current students also unlock their future by leveraging their creativity.
Website: https:/
About Dr Yohei Doi from Fujita Health University
Yohei Doi, M.D., Ph.D., is a Professor at the Departments of Microbiology and Infectious Diseases at Fujita Health University School of Medicine and the Director of the Center for Innovative Antimicrobial Therapy at the University of Pittsburgh. He has authored 226 publications and has served on several journals’ editorial boards. He is leading a clinical trial of the flu drug “Avigan” (favipiravir) as a potential treatment for COVID-19 at Fujita Health University.
About Dr Kentaro Tsukamoto from Fujita Health University
Kentaro Tsukamoto, D.V.M., Ph.D., is a Senior Assistant Professor in the Department of Microbiology at Fujita Health University School of Medicine. He has authored 24 publications. His areas of research interest include bacteriology and host-pathogen interaction.
Funding information
This work was supported by JSPS KAKENHI Grant Number JP19K07548 (K.T.), MEXT-Supported Program for the Strategic Research Foundation at Private Universities from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (H. Kurahashi), and the Grant for Joint Research Project of the Research Institute for Microbial Diseases, Osaka University (K.T.).
Media Contact
Dr Kentaro Tsukamoto
[email protected]
Related Journal Article
http://dx.




