Researchers create an animal model for studying GPI anchor deficiencies
Credit: © Miguel Rodriguez de los Santos
Impaired intelligence, movement disorders and developmental delays are typical for a group of rare diseases that belong to GPI anchor deficiencies. Researchers from the University of Bonn and the Max Planck Institute for Molecular Genetics used genetic engineering methods to create a mouse that mimics these patients very well. Studies in this animal model suggest that in GPI anchor deficiencies, a gene mutation impairs the transmission of stimuli at the synapses in the brain. This may explain the impairments associated with the disease. The results are now published in the journal “Proceedings of the National Academy of Sciences of the United States of America (PNAS)”.
Just as ships anchor to the seabed in storms and waves, GPI anchors (GPI = glycosylphosphatidylinositol) ensure that special proteins can hold on to the outside of living cells. If the GPI anchor does not function properly due to a gene mutation, the signal transmission and transport between cells are disrupted. “GPI anchor deficiencies comprise a group of rare diseases that primarily cause intellectual deficits and developmental delays,” explains Prof. Dr. Peter Krawitz from the Institute for Genomic Statistics and Bioinformatics at the University Hospital Bonn, who started his research at the Charité – Universitätsmedizin Berlin and continued it at the University Hospital Bonn. About 20 to 30 genes can be altered in GPI anchor deficiency.
A mutation in the PIGV gene was found in most European patients. It encodes an enzyme that is of great importance for the synthesis of the GPI anchor. Using the CRISPR-Cas9 gene scissors, the researchers and their colleagues from the Max-Planck-Institute for Molecular Genetics in Berlin modified the PIGV gene in mice based on a model of the patients. “Extensive behavioral tests have shown that this mouse model very closely reflects the disease observed in humans,” says Miguel Rodríguez de los Santos from the Institute for Medical Genetics and Human Genetics at the Charité. He has been working with Prof. Krawitz for years and is now continuing his research at the University Hospital Bonn.
How similar is the mouse to human patients?
The behavioral tests on the genetically modified mice were carried out in cooperation with scientists from the “Animal Outcome Core Facility” of the NeuroCure Cluster of Excellence at the Charité. The animals exhibited cognitive deficits, just like the patients. For example, they showed significantly worse spatial orientation than mice without this mutation and displayed altered social behavior. “They were particularly sociable, which is something we did not expect,” reports Rodríguez de los Santos.
Research revealed that patients with GPI anchor deficiencies also exhibit this sociability in some cases. The PIGV-altered mice also displayed deviations in the day-night rhythm. “This symptom has so far not appeared to be relevant, but it is certainly described in patients,” says Krawitz. “We now have the rare case that the large similarity of a mouse model allows us to infer and re-evaluate the symptoms of patients in reverse.”
Dysregulations in the hippocampus
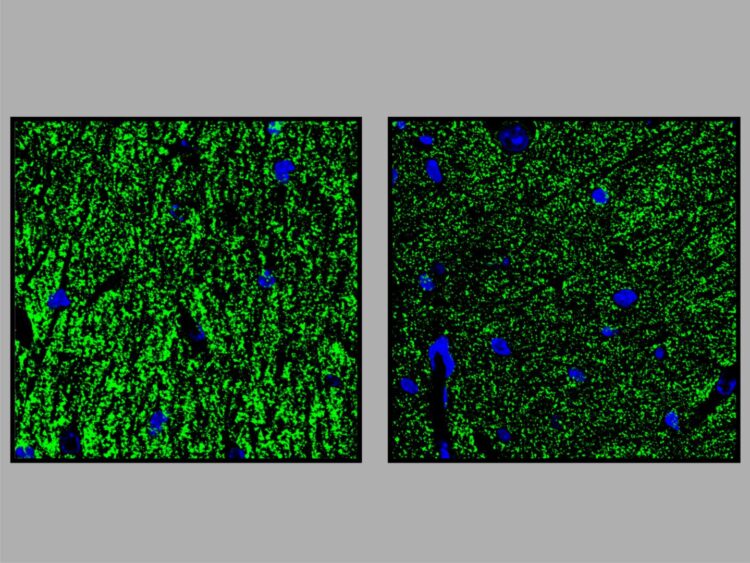
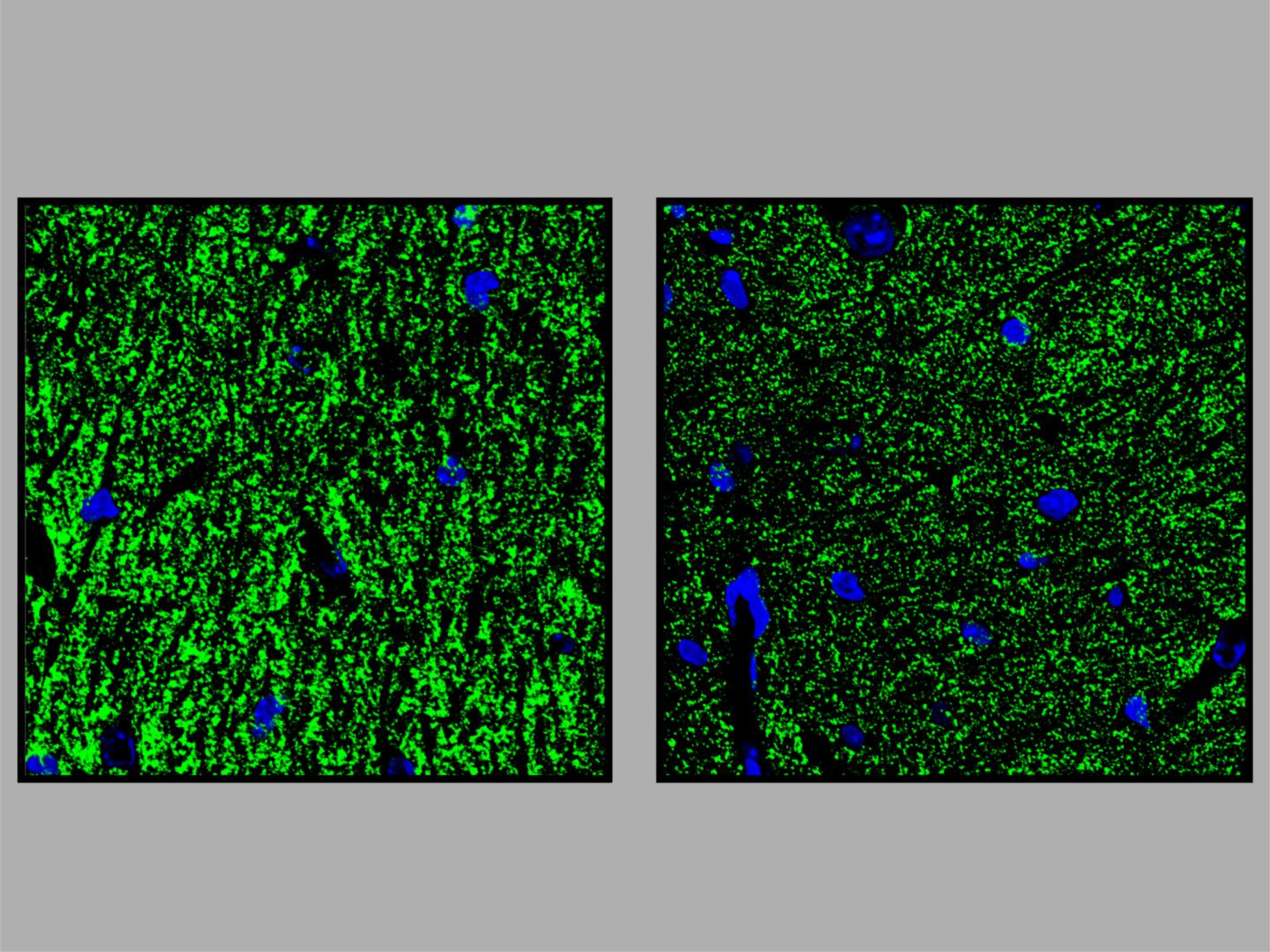
The researchers knew from preliminary studies that the hippocampus plays a major role in GPI anchor deficiencies. This brain structure, which resembles the shape of a seahorse, enables access to memories. The researchers studied microglia cells and subicular neurons from the hippocampus of genetically modified mice. Microglia cells are immune cells of the brain that fend off intruders. The subicular neurons are also responsible for memory retrieval. “Many genes in these two cell types were misregulated,” says Rodríguez de los Santos. This could explain why the mice showed orientation problems in the tests.
Researchers at the Neuroscience Research Center of the Charité studied the electric fields in the hippocampus of genetically modified mice. “This showed that the transmission of stimuli at the synapses was impaired,” says Krawitz. The results in the animal model suggest that the intellectual deficits found in the patients may be related to this synapse defect. Together with colleagues involved in translational epilepsy research at the University Hospital Bonn under Prof. Dr. Albert Becker, the researchers also discovered that the transgenic mice have an increased susceptibility to epilepsy. A further similarity with humans: Approximately 70 percent of patients with GPI anchor deficiencies develop epilepsy, again possibly caused by a synapse defect. Krawitz: “These observations and our mouse model open up completely new possibilities for further research in this direction.”
###
Participating institutions
In addition to the University of Bonn, the study included the Charité – Universitätsmedizin Berlin, the Berlin-Brandenburg School for Regenerative Therapies Berlin, the Max Planck Institute for Molecular Genetics, the University of Zurich, the Humboldt University of Berlin, The Jackson Laboratory for Genomic Medicine, Connecticut, University of Connecticut and the University Medical Center Göttingen.
Publication: Miguel Rodríguez de los Santos, Marion Rivalan, Friederike S. David, Alexander Stumpf, Julika Pitsch, Despina Tsortouktzidis, Laura Moreno Velasquez, Anne Voigt, Daniele Mattei, Melissa Long, Guido Vogt, Alexej Knaus, Björn Fischer-Zirnsak, Lars Wittler, Bernd Timmermann, Peter N. Robinson, Denise Horn, Stefan Mundlos, Uwe Kornak, Albert J. Becker, Dietmar Schmitz, York Winter, Peter M. Krawitz: A CRISPR-Cas9-engineered mouse model for GPI anchor deficiency mirrors human phenotype and exhibits hippocampal synaptic dysfunctions, Proceedings of the National Academy of Sciences of the United States of America (PNAS), DOI: 10.1073/pnas.2014481118
Media Contact
Peter Krawitz
[email protected]
Original Source
https:/
Related Journal Article
http://dx.