A new digital platform called Research Integrated Network of Systems (RINS) facilitates information sharing about clinical studies across disparate systems.
Credit: Illustration by Danielle Hutchison.
Academic medical centers continuously strive to enhance patient care. One of the major mechanisms to improve patient health outcomes is through translational research – bringing research breakthroughs from the lab to patients via clinical trials. Making clinical trials more efficient, and ultimately more successful, would significantly advance patient care. However, fragmentation of the relevant data necessary to implement improvements to translational science is a significant barrier.
While some bioinformatic tools have attempted to address this problem, they often lacked the ability to assess the efficiency of translational science. Researchers at the South Carolina Clinical & Translational Research (SCTR) Institute, a Clinical and Translational Science Awards (CTSA) hub with an academic home at the Medical University of South Carolina (MUSC), have developed a novel bioinformatic tool called RINS, the Research Integrated Network of Systems, that can be used to evaluate whether improvements to the clinical trial process are making a difference. Their results, published online on March 17 in the Journal of the American Medical Informatics Association, showed that RINS can integrate data about clinical studies across disparate systems and provide metrics about MUSC’s clinical trial efficiency and effectiveness.
“You could call it meta-data – it’s data about how we produce data in studies or how we get studies up and running and accelerate them,” said Leslie A. Lenert, M.D., chief research information officer for MUSC and director of the Biomedical Informatics Center (BMIC). “Those were all in a different place, in a different format, using numbers that didn’t have an agreed-upon definition. But we brought it all into one view to get a comprehensive look at the processes for doing science. And we’ve never had that before because the data lived in disparate systems.”
Making informed decisions about how best to improve and streamline translational research practices requires detailed data, and to accumulate that data, institutions need strong evaluative tools. To that end, MUSC has developed and utilized a research transaction management system, SPARCRequest, that provides a powerful tool to help researchers determine budgets for grant applications, navigate the regulatory process, recruit diverse study participants and conduct clinical trials. As an open-source platform, SPARCRequest is utilized by 12 CTSA and Clinical and Translation Research (CTR) hubs comprising 27 institutions across the country.
While SPARCRequest was instrumental in streamlining clinical research, it wasn’t originally set up for metric tracking across disparate systems. To address that problem, Lenert approached Royce R. Sampson, chief operating officer for SCTR and one of the inventors of SPARCRequest, with an idea to generate a research data mart. The data mart, envisioned by Lenert, took the research metric-tracking activities that Sampson was already leading with SPARCRequest to the next level and enabled cross-disciplinary group tracking and communication.
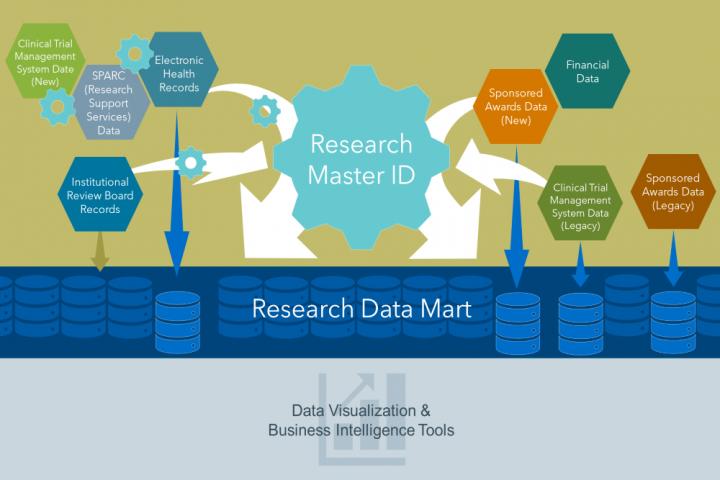
“A research data mart is a data warehouse that integrates data from multiple systems into a format that makes it easier to return metrics and analyses from different systems,” said Katie G. Kirchoff, database administrator who runs the research data mart for BMIC.
“It’s a simple concept, but hard to execute,” added Lenert, who also serves as assistant provost for Data Sciences and Informatics. “No one’s had the opportunity to bring together these diverse systems to tell us how long it takes an institutional review board protocol to get approved or whether this funding led to that outcome – those are difficult things to do.”
In order to house and access the data within the warehouse, the RINS team needed a way to identify each study uniquely and link the disparate systems together. To solve the problem on identifying each study, the team created a research master identifier (RMID).
“RMID helped us be able to have a standardized protocol numbering system so we could link the systems that we have in-house,” said Sampson. “RINS is the platform where we pull it all together.”
Connecting the various systems together was achieved through the development of application programming interfaces (APIs). The APIs bridge the systems together and enable data to be updated across systems in near real-time.
Using RMIDs and APIs, RINS integrates SPARCRequest with other electronic databases, including MUSC’s electronic health record; electronic institutional review board; and systems for grants award management, expenditure tracking and clinical trial management. RINS has been sufficiently flexible to accommodate new programs while also maintaining historical data.
As a new digital data warehouse, RINS provides two key improvements to monitoring translational science data. The first improvement is the integration of disparate systems onto one platform. This allows for a more complete picture of research processes and identifies the successes and challenges of the clinical trials.
The second improvement is the use of a decentralized system. Most platforms use a centralized system that would require each area of research administration to adopt whatever system had been integrated into the centralized solution, even if it was not the one best suited to its needs. In contrast, RINS is a decentralized system that enables each group to use its preferred system while maintaining comprehensive, integrated reporting. This allows clinical studies, for example, to be tracked across systems to give a much clearer idea of the life cycle of the project, provides metrics about the impact of translational efforts and identifies areas to improve clinical trial efficiency.
“We’re not trying to build a system that fits all needs, but we’re actually utilizing existing systems and linking them together for reporting needs,” said Wenjun He, a systems engineer at SCTR who is the lead author for the report. “We can always expand it to any new system around campus – it’s a seamless transition.”
As more institutions look at how their programs perform, and as more funding agencies require performance data, institutions may look to RINS for help. While RINS is an open-source platform at MUSC, it could be difficult for other institutions to adopt the platform as is.
“It is customized, at this point, to systems that we utilize,” said Andrew M. Cates, director of Research Web Solutions. “New integrations could be made, but another institution can’t start using it without having to make some customizations or have systems similar to what we have at MUSC.”
Although not currently available to all institutions, RINS has sparked interest from the SPARCRequest Open Source Consortium and may help to transition these institutions to better metric monitoring. Because many of these institutions already employ SPARC, it might be easier for them to integrate a broader range of systems through RINS.
Working together across a broad range of systems helps to ensure that clinical trials run smoothly and effectively. Through tools such as RINS and a wealth of services and resources, SCTR supports investigators in conducting effective research.
“The goal is to improve the performance of trials and study teams and remove barriers and challenges to clinical research,” said Sampson. “SCTR provides over 900 consults, services and resources per year to support and educate study teams. RINS enables our ability to identify opportunities at an institutional level.”
Overall, RINS is a powerful tool for extracting critical data on translational science. RINS integrates data from clinical studies across disparate systems and provides analyses that can be used to improve the clinical trial process. The Office of Clinical Research has been an integral, critical and leading stakeholder in RINS and with their continued support, the future of RINS could incorporate data from the basic sciences to provide new data on awarding research grants, core facilities and groundbreaking science.
###
About MUSC
Founded in 1824 in Charleston, MUSC is the oldest medical school in the South as well as the state’s only integrated academic health sciences center, with a unique charge to serve the state through education, research and patient care. Each year, MUSC educates and trains more than 3,000 students and nearly 800 residents in six colleges: Dental Medicine, Graduate Studies, Health Professions, Medicine, Nursing and Pharmacy. MUSC brought in more than $271 million in biomedical research funds in fiscal year 2020, continuing to lead the state in obtaining National Institutes of Health funding, with more than $129.9 million. For information on academic programs, visit musc.edu.
As the clinical health system of the Medical University of South Carolina, MUSC Health is dedicated to delivering the highest quality patient care available while training generations of competent, compassionate health care providers to serve the people of South Carolina and beyond. Comprising some 1,600 beds, more than 100 outreach sites, the MUSC College of Medicine, the physicians’ practice plan and nearly 275 telehealth locations, MUSC Health owns and operates eight hospitals situated in Charleston, Chester, Florence, Lancaster and Marion counties. In 2020, for the sixth consecutive year, U.S. News & World Report named MUSC Health the No. 1 hospital in South Carolina. To learn more about clinical patient services, visit muschealth.org.
MUSC and its affiliates have collective annual budgets of $3.2 billion. The more than 17,000 MUSC team members include world-class faculty, physicians, specialty providers and scientists who deliver groundbreaking education, research, technology and patient care.
About the SCTR Institute
The South Carolina Clinical & Translational Research (SCTR) Institute is the catalyst for changing the culture of biomedical research, facilitating the sharing of resources and expertise and streamlining research-related processes to bring about large-scale change in clinical and translational research efforts in South Carolina. Our vision is to improve health outcomes and quality of life for the population through discoveries translated into evidence-based practice. To learn more, visit https:/
Media Contact
Heather Woolwine
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




