A groundbreaking advancement in brain imaging technology promises to revolutionize the way clinicians and researchers understand brain metabolism and related diseases. Researchers at the University of Illinois Urbana-Champaign have developed a novel method that combines high-speed magnetic resonance imaging (MRI) with sophisticated machine learning algorithms to capture detailed metabolic activity across the entire brain. This innovation enables non-invasive, high-resolution metabolic imaging in a fraction of the time previously required, yielding new insights into disorders such as brain tumors and multiple sclerosis.
Traditional MRI methods have long excelled in providing detailed structural images of the brain, allowing doctors to visualize anatomical features and abnormalities. Functional MRI (fMRI), another widely used technique, enhances this by detecting blood flow and oxygenation changes associated with neural activity. Despite their widespread use, neither technique can directly measure the metabolic processes that underpin brain function and dysfunction. Metabolic imaging offers the potential to detect neurochemical changes that precede, and may even predict, pathology—a capability that is now within reach thanks to this remarkable technological leap.
The new approach hinges on magnetic resonance spectroscopic imaging (MRSI), a modality that captures signals not only from water molecules, like conventional MRI, but from a spectrum of brain metabolites and neurotransmitters. These molecular signals provide a direct window into brain metabolism, which is crucial for understanding a myriad of neurological conditions. However, MRSI has been hampered historically by two major constraints: long acquisition times and poor signal quality obscured by noise. The Illinois team has addressed both challenges simultaneously by integrating ultrafast data acquisition techniques with advanced physics-informed machine learning data processing. This harmonious combination achieves metabolic brain imaging within approximately 12 and a half minutes—a timeframe compatible with clinical workflow and patient comfort.
.adsslot_plDSdtzMHf{width:728px !important;height:90px !important;}
@media(max-width:1199px){ .adsslot_plDSdtzMHf{width:468px !important;height:60px !important;}
}
@media(max-width:767px){ .adsslot_plDSdtzMHf{width:320px !important;height:50px !important;}
}
ADVERTISEMENT
The research team, led by Professor Zhi-Pei Liang of the University of Illinois’ Beckman Institute for Advanced Science and Technology, has published these findings in Nature Biomedical Engineering. Their work demonstrates that the new MRSI technique not only significantly reduces scan times but also improves the spatial resolution and signal specificity of metabolic imaging. This breakthrough allows for whole-brain metabolic mapping at a level of detail never before achieved in a clinical setting, opening vast opportunities for personalized medicine and early intervention strategies.
Application of this advanced imaging technique has unveiled striking metabolic heterogeneity among different regions of the healthy brain. Contrary to the assumption that the brain’s chemical activity is fairly uniform, the team found distinct patterns of metabolite distributions and neurotransmitter activity that vary regionally. This nuanced understanding of baseline brain metabolism lays the foundation for identifying subtle deviations associated with disease states, potentially allowing clinicians to distinguish pathological changes from normal variation more reliably.
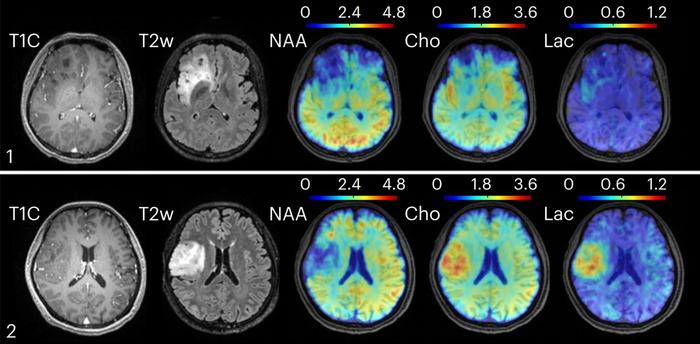
In exploring pathological conditions, the researchers applied their method to patients with oligodendroglioma brain tumors of various grades. Remarkably, while conventional clinical MRI images failed to differentiate between grade II and grade III tumors, metabolic imaging revealed elevated choline and lactate levels in the more aggressive grade III lesions. This metabolic distinction, invisible to standard imaging, carries tremendous diagnostic and prognostic importance, potentially guiding more targeted therapeutic decisions.
Moreover, in a separate cohort of multiple sclerosis (MS) patients, the metabolic imaging technique detected molecular changes related to neuroinflammation and neuronal dysfunction up to 70 days before these alterations became apparent on conventional MRI scans. Early identification of such metabolic disturbances could transform MS management, enabling timely intervention before irreversible damage occurs, thereby improving patient outcomes and quality of life.
The integration of machine learning algorithms into the imaging pipeline was pivotal in addressing MRSI’s previous limitations. These algorithms leverage physical models of the MRI acquisition process and the underlying metabolic spectra, significantly reducing noise and artifacts in the reconstructed images. The synergy of rapid data capture and advanced computational processing results in high-fidelity metabolic images that maintain clinical relevance without compromising speed or resolution.
From a clinical perspective, the potential implications of this technology are profound. Not only does it provide clinicians with a new dimension of metabolic information for diagnosis and disease monitoring, but it also offers avenues for personalized medicine. By tracking changes in metabolic profiles over time, physicians can evaluate the effectiveness of therapeutic regimens more sensitively and adjust treatments to align with the patient’s unique biochemical brain environment.
Historically, the vision for metabolic brain imaging was pioneered by Nobel laureate Paul Lauterbur, whose foundational work in MRI paved the way for contemporary imaging modalities. Despite the promise of metabolic MRI, technological constraints have long impeded its translation into clinical practice. The current breakthrough fulfills Lauterbur’s foresight by delivering fast, high-resolution metabolic brain imaging accessible via standard clinical MRI machines, thereby bridging the gap between research innovation and medical application.
As healthcare moves steadily towards a model emphasizing personalized, predictive, and precision medicine, technologies like ultrafast MRSI stand to become invaluable tools. Their ability to noninvasively visualize metabolic alterations provides an urgently needed approach to managing neurological diseases, many of which involve early metabolic perturbations that currently go undetected. The scalability and relatively short scan times further ensure that such techniques can be integrated into routine clinical workflows without causing patient burden.
Looking ahead, the research team is poised to expand applications of their technology beyond brain tumors and multiple sclerosis to include a wider spectrum of neurological disorders. These include neurodegenerative diseases such as Alzheimer’s and Parkinson’s, epilepsy, and psychiatric conditions where metabolic dysregulation plays a critical role. Continued refinement of the acquisition and processing methods may yield even faster scans, sharper images, and broader metabolic characterization capabilities.
Although in its early stages of clinical deployment, the promise of ultrafast metabolic brain imaging is undeniable. This technological milestone not only augments the capabilities of conventional MRI but also ushers in a new era in neuroimaging—one capable of revealing the biochemical underpinnings of brain health and disease with unprecedented clarity and speed. The integration of this method into clinical practice holds the potential to transform diagnostics, treatment decision-making, and research in neurology and psychiatry, fundamentally changing how brain disorders are understood and managed.
For more information and inquiries about this technology, Dr. Zhi-Pei Liang welcomes correspondence at [email protected]. The full research article titled “Ultrafast J-resolved magnetic resonance spectroscopic imaging for high-resolution metabolic brain imaging” was published on June 20, 2025, in Nature Biomedical Engineering. The work was generously supported by the Arnold and Mabel Beckman Foundation, underscoring the collaborative commitment to advancing medical imaging innovation.
Subject of Research: People
Article Title: Ultrafast J-resolved magnetic resonance spectroscopic imaging for high-resolution metabolic brain imaging
News Publication Date: 20-Jun-2025
Web References: https://www.nature.com/articles/s41551-025-01418-4
References: DOI: 10.1038/s41551-025-01418-4
Image Credits: Yibo Zhao, University of Illinois
Keywords: Magnetic Resonance Imaging, Magnetic Resonance Spectroscopic Imaging, Brain Metabolism, Machine Learning, Oligodendroglioma, Multiple Sclerosis, Neuroimaging, Metabolic Brain Imaging, High-Resolution MRI, Neuroinflammation, Personalized Medicine, Ultrafast MRI
Tags: advanced magnetic resonance spectroscopic imagingbrain disorders diagnosisbrain metabolism imagingbrain tumors imaginghigh-resolution MRI technologyinnovative MRI techniquesmachine learning in medical imagingmetabolic activity mappingmultiple sclerosis researchneurochemical changes detectionnon-invasive brain imagingpredictive pathology imaging