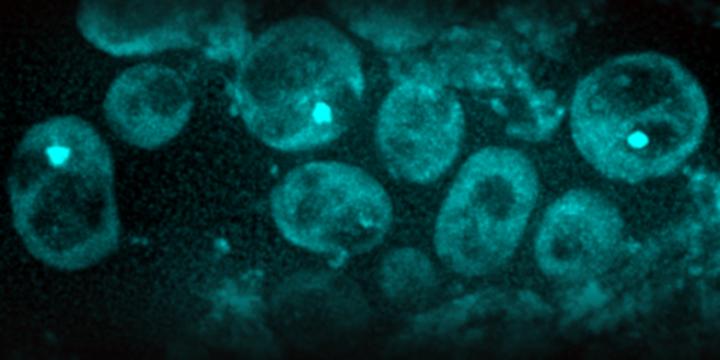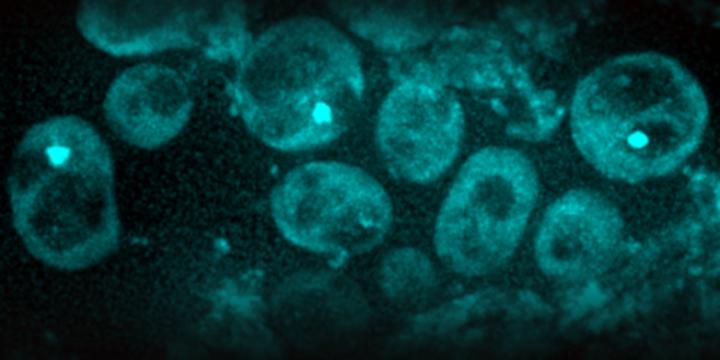
IMAGE: Scientists found in yeast cells protein aggregates (light green spots). As cells age and divide, these aggregates become more frequent (micrograph).
Credit: ETH Zurich / Juha Saarikangas
We age because the cells in our bodies begin to malfunction over the years. This is the general view that scientists hold of the ageing process. For example, in older people the cells' internal quality control breaks down. This control function usually eliminates proteins that have become unstable and lost their normal three-dimensional structure. These deformed proteins accumulate in the cells in a number of diseases, such as Parkinson's and Alzheimer's.
For Yves Barral, Professor of Biochemistry at ETH Zurich, the view of the ageing process as a consequence of flawed cell function and disease is too narrow. It ignores the fact that the mentioned so-called prion-like protein accumulations could have a positive effect, too, and therefore should not be referred to as cellular malfunction, he says.
Old cells cope better with stress
Barral drew this conclusion based on his research on yeast cells. He and his colleagues recently found in these cells a new type of protein aggregate, which appears as the cells get older. As the scientists were able to show, these protein aggregates do not arise as the result of a cell's malfunctioning internal quality control. On the contrary: in yeast cells with such aggregates, quality control functions even better. "It certainly seems that these aggregates help yeast cells to cope with the physiological changes caused by ageing," says Juha Saarikangas, a postdoc in Barral's group and first author of the recent study in the journal eLife. "We are very exited to learn what type of information is stored in these structures."
The scientists assume that these age-associated aggregates are formed by several different proteins. The researchers have already identified one prion-like protein that is part of the accumulations. What other proteins are involved and why the aggregates remain in the parent cells during cell division are subjects of further research.
Aggregates improve memory
Only in recent years have scientists speculated that aggregating proteins in the cells can generally play a positive role. Barral and his research group showed back in 2013 that yeast cells memorise experiences related to unsuccessful sexual reproduction attempts in the form of aggregated proteins (see ETH News from 05.12.2013, https://www.ethz.ch/en/news-and-events/eth-news/news/2013/12/protein-clumps-as-memory.html). These aggregates – which are not identical to the newly discovered age-associated accumulations – thus serve as molecular memory for yeast cells. Even in mice there is a positive relationship between prion-like aggregates and memory. A few months ago, American scientists demonstrated that mice with such accumulations in their nerve cells exhibit a more stable long-term memory.
"Bad end to a good thing"
Whether such age-associated protein accumulations are primarily a malfunction or a normal function of healthy cells is for Barral a scientific question – one in which philosophy also plays a role: "Our western society understands ageing as something that is predominantly negative, a disease that has to be combated," he says. "This thinking is reflected in the work of many scientists, whose research on ageing focuses on finding defects in cells." Other societies, however, place more value on the positive effects of ageing, such as increased experience and knowledge – a view that corresponds with the newly discovered role of aggregates as information storage or memory for cells.
"We're still a fairly small group of scientists who say: aggregate proteins are not pathological – they are neither an accident nor a defect," says Barral. Rather, these proteins aggregate because it is their normal function. Diseases such as Parkinson's and Alzheimer's only arise when the system becomes imbalanced and too many prion-like proteins accumulate in the wrong place in the cells. Barral continues: "There are two aspects to ageing. Yes, you die at the end of the process, and this is negative. But you die wise. And Alzheimer's is perhaps a bad end to a good thing."
###
Reference
Saarikangas J, Barral Y: Protein aggregates are associated with replicative aging without compromising protein quality control. eLife 2015, e06197, doi: 10.7554/eLife.06197 [http://dx.doi.org/10.7554/eLife.06197]
Media Contact
Yves Barral
[email protected]
41-446-320-678
@ETH_en
http://www.ethz.ch/index_EN