Credit: HIROKI IRIEDA, SHINSHU UNIVERSITY
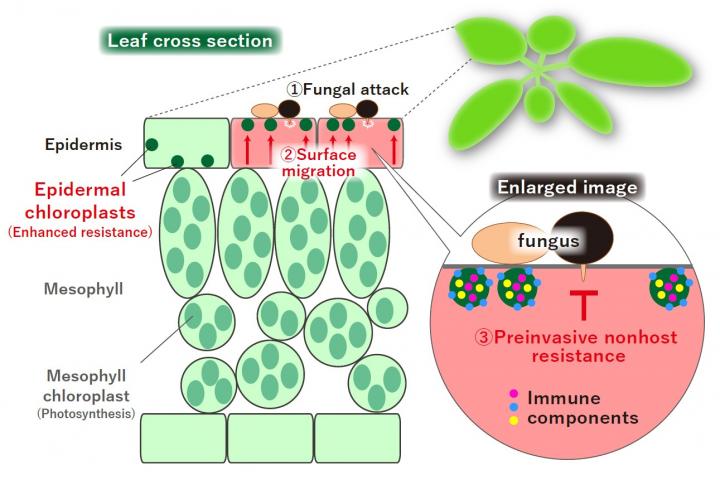
It is said that 10 to 15% of the world’s agricultural production loss is caused by diseases, which is equivalent of the food for about 500 million people. And since 70-80% of this plant disease is caused by filamentous fungi, protecting crops from filamentous fungi is an important issue in effectively feeding the world population. In order for pathogenic fungi to infect plants, they must break through the epidermal cells of the plant and invade the interior. In other words, plant epidermal cells act as the first barrier to stop the attack of pathogenic fungi in the environment. So what kind of defense functions do epidermal cells have?
Interestingly, it was known that the epidermis of plants contain small chloroplasts that are not so involved in photosynthesis. However, it was unclear what function it had. Why are there small chloroplasts in the epidermis of plants that do not contribute much to photosynthesis?
Assistant Professor Hiroki Irieda of the Faculty of Agriculture, Shinshu University and Professor Yoshitaka Takano, Graduate School of Agriculture, Kyoto University, found that small chloroplasts in the epidermis of plants control the entry of fungal pathogens. The duo discovered that the small chloroplasts move inside the cell dramatically to the surface layer in response to the fungal attack and is involved in such defense response. Furthermore, the duo found that multiple immune factors involved in the defense response of plants are specifically found in the epidermal chloroplast, which contributes to the enhancement of resistance to the invasion of pathogen filamentous fungi. Based on this research, they hope to develop crop protection technology to enhance and control the chloroplast function of the plant epidermis, which functions as a barrier against foreign invaders, and to increase the immunity of plants, which will hopefully lead to reduction of disease damage and improvement of food production.
In this study, the duo first investigated what kind of pathogenic fungi the epidermal chloroplasts respond to. As a result, they found that multiple pathogenic filamentous fungi cause surface migration of epidermal chloroplasts. Interestingly, it was also found that these pathogenic filamentous fungi were the so-called “nonadapted” and were blocked from invading epidermal cells. On the other hand, adapted pathogenic fungus is more likely to invade the epidermis in plants in which the epidermal chloroplasts have stopped migrating to the surface layer.
Next, they succeeded in finding plant proteins involved in the surface migration of epidermal chloroplasts. When a plant that overproduces this protein was created by gene transferintroduction, epidermal chloroplasts did not move to the surface layer against pathogenic filamentous fungi. In this transgenic plant, the immunity to the epidermal invasion of pathogenic filamentous fungi is reduced. It was also found that pathogenic fungi are more likely to invade the epidermis in plants in which the epidermal chloroplasts have stopped migrating to the surface layer. These results indicate that the migration of epidermal chloroplasts to the surface layer is involved in the defense response that blocks the invasion of pathogenic fungi.
Furthermore, it was revealed that multiple factors involved in plant immunity are specifically found in the epidermal chloroplast. In addition, in mutant plants in which these types of immune factors that are found in epidermal chloroplasts did not function, migration of epidermal chloroplasts to the surface layer was confirmed, but the invasion rate of pathogenic fungi increased.
In plants that suppress the intracellular migration of epidermal chloroplast, the immunity to the epidermal invasion of pathogenic filamentous fungi is reduced. Based on the results of this study, the duo hopes to develop technology to enhance and control the function of epidermal chloroplasts, such as increasing the intracellular migration efficiency of epidermal chloroplasts when attacked by pathogenic fungi filamentous fungi.
###
Acknowledgements
We thank Paul Schulze-Lefert for pen2-1 and pen2-1 with PEN2-GFP, Roger W. Innes for edr1-1, Yasuhiro Kadota for bik1 pbl1, Yube Yamaguchi for pepr1-1 pepr2-1, Volker Lipka for sid2-2, and Masamitsu Wada and Sam-Geun Kong for the anti-CHUP1 antibody. We thank Daisuke Shiomi for his technical assistance and helpful discussions. We thank Kei Hiruma, Asuka Hagiwara, Kazuho Takesue, Kosei Shiratori, and Hideki Baba for their technical assistance. We thank Katsuharu Saito for his help with statistical analyses. We also thank the Research Center for Supports to Advanced Science, Shinshu University for use of facilities. This work was supported by JSPS KAKENHI Grant Numbers 15K18648 (to H.I.), 18K05643 (to H.I.), and 18H02204 (to Y.T.), by the Asahi Glass Foundation (to Y.T.), and by the Leading Initiative for Excellent Young Researchers (LEADER) program of MEXT (to H.I.).
Media Contact
Hitomi Thompson
[email protected]
Related Journal Article
http://dx.




