In a groundbreaking study published in the Journal of Cancer Research and Clinical Oncology, researchers Zhao et al. unveil the intricate relationship between cancer-associated adipocytes (CAA) and breast cancer progression. This novel research sheds light on how CAA-derived interleukin-6 (IL-6) plays a crucial role in promoting programmed death-ligand 1 (PD-L1) expression, a key player in immune evasion by tumors, through the activation of the STAT3/miR-497a-5p signaling pathway. The findings could signify a monumental step in understanding breast cancer’s molecular environment and its implications for therapeutic interventions.
Breast cancer remains one of the most pervasive malignancies among women globally. It is characterized by a wide array of biological behaviors and responses to therapy. Understanding the interplay between tumor cells and their microenvironment is essential for unveiling new therapeutic targets. Zhao and his team delve deeply into the role of adipocytes—fat cells that are not merely storage units but active participants in tumor biology.
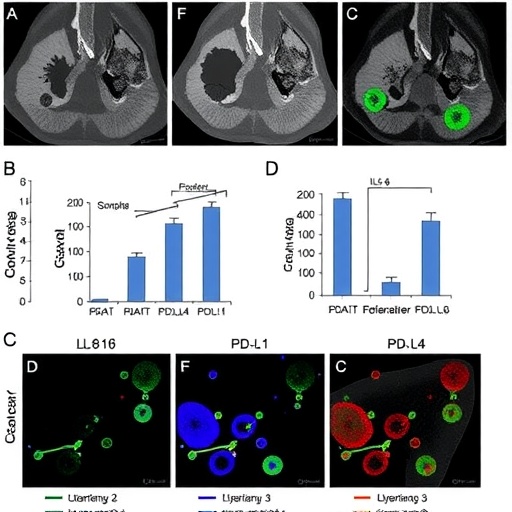
The study meticulously demonstrates that CAA-derived IL-6 is a potent promoter of PD-L1 expression in breast cancer cells. This discovery is significant because PD-L1 is known to inhibit T-cell activity, allowing tumors to escape immune surveillance. By elucidating the mechanisms underpinning this process, the research opens the door to innovative therapeutic strategies aimed at disrupting this communication.
At the molecular level, the activation of the STAT3 (Signal Transducer and Activator of Transcription 3) pathway emerges as a critical mediator in this interaction. The study confirms that IL-6 activates STAT3, leading to increased expression of PD-L1 in breast cancer cells. This finding reveals new dimensions in the understanding of how immune evasion mechanisms operate in breast cancer, highlighting STAT3 as a possible therapeutic target.
Moreover, the study implicates the microRNA miR-497a-5p in this signaling cascade. As the researchers unravel the complexities of the interplay between IL-6 and miR-497a-5p, they provide evidence that the modulation of miR-497a-5p affects PD-L1 levels in cancer cells. Such insights emphasize the multifaceted roles of microRNAs in cancer biology, particularly in the context of immune modulation.
From a broader perspective, this research underscores the importance of the tumor microenvironment in shaping tumor behavior and responses to treatment. By focusing on the interplay between adipocytes and cancer cells, the researchers illuminate a previously underappreciated aspect of tumor biology. This knowledge could lead to novel approaches that reprogram the tumor microenvironment, thereby enhancing anti-tumor immunity.
For clinicians and researchers dedicated to breast cancer, the implications of this study cannot be overstated. By targeting the IL-6/STAT3/miR-497a-5p axis, it may be possible to devise new treatments that thwart PD-L1 upregulation, potentially reversing immune evasion in tumors. This research offers a promising avenue for developing combination therapies that incorporate immunotherapy with agents targeting the adipocyte-cancer cell interaction.
Furthermore, the study raises questions about the role of obesity and metabolic health in breast cancer progression. Given that adipose tissue produces a variety of inflammatory cytokines, researchers can explore how lifestyle and metabolic factors may influence breast cancer risk through their effects on CAA and IL-6 production. This connection between metabolic health and cancer biology is an exciting frontier for research, aligning with the growing recognition of cancer as a systemic disease.
This investigation also presents a compelling narrative about the necessity of personalized medicine in oncology. Understanding the unique microenvironmental factors influencing each patient’s tumor could lead to tailored therapeutic approaches, ultimately improving patient outcomes. The identification of biomarkers associated with IL-6 and PD-L1 expression could pave the way for better predictive models in breast cancer.
As the oncological community absorbs these revelations, it establishes a foundation for future investigations. Upcoming studies could explore the therapeutic potential of IL-6 inhibitors or STAT3 antagonists in the context of breast cancer. Additionally, the role of miR-497a-5p could be dissected further to explore its applicability as a biomarker or therapeutic target.
These findings not only advance our comprehension of breast cancer biology but also challenge us to reconsider the strategies employed in cancer treatment. The discussion around adiposity’s impact on cancer progression calls for a holistic approach, integrating cancer research with nutrition and public health initiatives.
This research by Zhao et al. is a potent reminder of the complexities inherent within cancer biology and the necessity for continued exploration of various signaling pathways and their implications in tumor development. The intersection of immune evasion and metabolism could offer critical insights leading to revolutionary breakthroughs in cancer therapeutics.
Finally, as the medical community reflects on the implications of this study, the hope is that it will catalyze discussions regarding innovative treatment modalities that prioritize modulating the tumor microenvironment. With continued investment in cancer research, the dream of improving survival rates and quality of life for breast cancer patients moves closer to reality, fueled by advances in understanding the multifaceted interactions that define cancer progression.
Subject of Research: Breast cancer and its microenvironmental interaction with adipocytes
Article Title: CAA-derived IL-6 promoted the PD-L1 expression of breast cancer via STAT3/miR-497a-5p signaling.
Article References:
Zhao, C., Zhou, X., Li, X. et al. CAA-derived IL-6 promoted the PD-L1 expression of breast cancer via STAT3/miR-497a-5p signaling.
J Cancer Res Clin Oncol 151, 293 (2025). https://doi.org/10.1007/s00432-025-06324-5
Image Credits: AI Generated
DOI: 10.1007/s00432-025-06324-5
Keywords: IL-6, PD-L1, breast cancer, adipocytes, STAT3, miR-497a-5p, tumor microenvironment, immunotherapy, metabolic health.
Tags: adipocyte interaction with cancer cellsbreast cancer microenvironmentcancer research advancementscancer-associated adipocytes in tumor biologyIL-6 role in breast cancerimmune evasion in breast cancerinterleukin-6 and tumor progressionJournal of Cancer Research and Clinical Oncology findingsPD-L1 expression mechanismsprogrammed death-ligand 1 and immunotherapySTAT3 signaling pathway in tumorstherapeutic targets in breast cancer