Pancreatic ductal adenocarcinoma (PDAC) remains one of the deadliest forms of cancer, holding its position as the fourth leading cause of cancer-related deaths globally. This aggressive disease is characterized by its late diagnosis and the remarkable resistance to current therapeutic strategies, making early intervention and targeted treatment critical yet challenging. Recent insights into the molecular underpinnings of PDAC have painted a complex picture of its heterogeneity and adaptability, further complicating effective treatment. The latest research reveals a key player in this landscape: isoleucyl-tRNA synthetase 2 (IARS2).
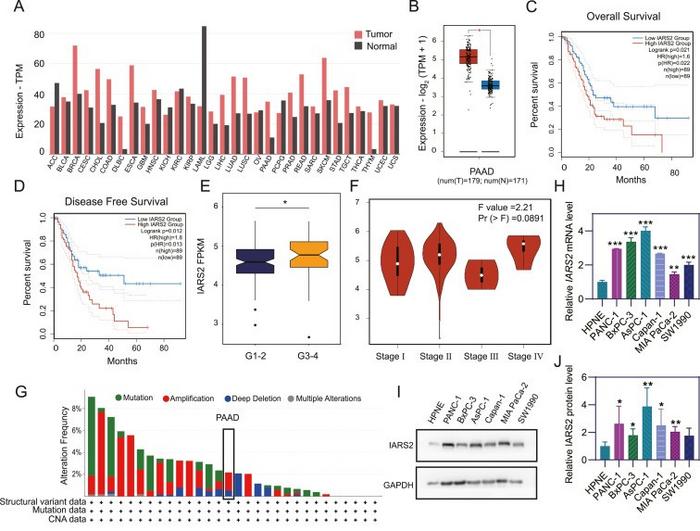
IARS2 has been identified as significantly upregulated in PDAC tissues and cell lines, suggesting its role in cancer progression. This finding is not merely academic; it connects IARS2 expression levels with patient outcomes. High levels of IARS2 in PDAC patients correlate with decreased overall survival and disease-free survival rates. This correlation presents a daunting yet enlightening opportunity for further exploration of IARS2 as a prognostic marker that could potentially shift clinical approaches towards this malignancy.
The researchers explore IARS2’s involvement in the disease’s aggressive nature. They postulate that the protein enhances not only cell proliferation but also the propensity for metastasis. Detailed molecular studies, including both in vitro and in vivo experiments, reveal that altering IARS2 expression significantly impacts the disease’s behavior. When researchers downregulated IARS2, they observed substantial decreases in proliferation, migration, and invasion of PDAC cells, establishing it as a vital contributor to the cancer’s aggressive phenotype.
The findings extend beyond just cellular behaviors; they delve deep into the molecular pathways that IARS2 influences. Transcriptomic analysis of pancreatic ductal adenocarcinoma cohorts highlights that IARS2 regulates various biological processes fundamental to cancer progression. In this context, gene set enrichment analysis (GSEA) underscored the activation of critical signaling pathways including WNT signaling, cell cycle regulation, and apoptotic pathways, particularly in the cohort with high IARS2 expression.
One of the most striking revelations from this research is the relationship between IARS2 expression and markers of cancer stemness. The study presented compelling evidence linking elevated IARS2 levels with the expression of key stemness markers such as CD44, MET, and CD133, insinuating that IARS2 plays a crucial role in maintaining a stem cell-like state within PDAC. This aspect is particularly alarming as it implies IARS2 may also contribute to the tumor’s ability to evade standard therapeutic interventions that target bulk tumor cells rather than their more resilient stem cell counterparts.
Moreover, the interaction of IARS2 with the immune landscape presents another layer of complexity. The study reveals that higher IARS2 levels correlate with reduced infiltration of CD8+ T cells, which are crucial for effective anti-tumor immunity. This suggests that IARS2 may not only facilitate tumor proliferation but could also contribute to creating an immunosuppressive microenvironment that supports tumor growth and progression, thus undermining the body’s immune response.
On a mechanistic level, IARS2 seemingly exerts its effects through the stabilization of β-catenin, a key protein in the Wnt signaling pathway. By preventing its phosphorylation-dependent degradation through interactions with β-TrCP, IARS2 contributes to the pathologic activation of the Wnt/β-catenin signaling cascade. This mechanism not only underscores IARS2’s role in promoting tumorigenesis but also positions it as a potential target for therapeutic intervention.
In conclusion, this study illuminates the critical involvement of IARS2 in pancreatic ductal adenocarcinoma, framing it as both a prognostic marker and a potential therapeutic target. The observed relationship between IARS2 levels, aggressive disease behavior, and poor patient prognosis underscores the urgency for new treatment strategies focused on pivotal molecular players like IARS2. However, this research acknowledges its limitations and calls for further investigation into the specific molecular interactions at play, particularly concerning how IARS2 might influence β-catenin degradation.
As research continues to unfold, it is imperative to focus on characterizing the full extent of IARS2’s role in PDAC. Such insights could lead to groundbreaking advancements in treatment approaches, potentially shifting the prognosis for one of the most challenging cancers to treat.
Subject of Research: Isoleucyl-tRNA synthetase 2 (IARS2) in pancreatic ductal adenocarcinoma
Article Title: Isoleucyl-tRNA synthetase 2 promotes pancreatic ductal adenocarcinoma proliferation and metastasis by stabilizing β-catenin
News Publication Date: 2024
Web References: https://www.sciencedirect.com/journal/genes-and-diseases
References: Isoleucyl-tRNA synthetase 2 promotes pancreatic ductal adenocarcinoma proliferation and metastasis by stabilizing β-catenin
Image Credits: Genes & Diseases
Keywords: Pancreatic cancer, IARS2, β-catenin, WNT pathway, cancer stemness, metastasis, prognosis, molecular targets.
Tags: advancements in cancer research methodologiescancer heterogeneity and adaptabilitycancer treatment resistanceearly intervention in pancreatic cancerIARS2 expression and patient outcomesIARS2 role in pancreatic cancermetastatic behavior in pancreatic cancermolecular mechanisms of PDACpancreatic ductal adenocarcinoma researchprognostic markers for cancersurvival rates in pancreatic cancertargeted therapies for PDAC