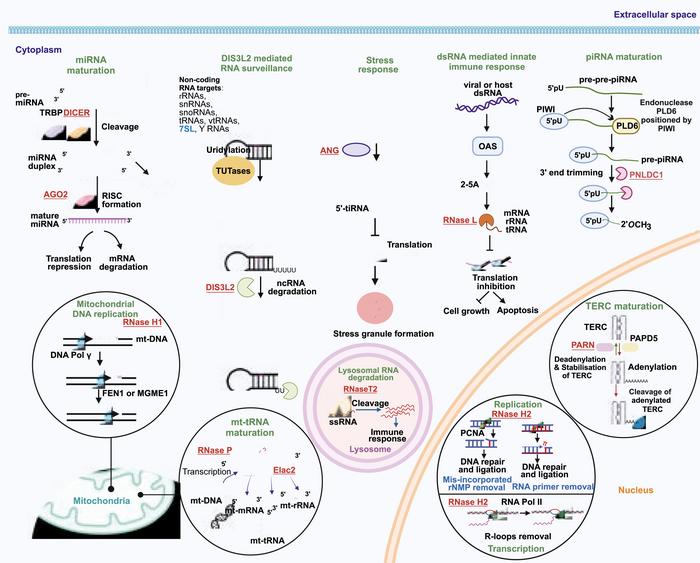
A groundbreaking review recently published in Genes & Diseases has unveiled the critical and multifaceted role that ribonucleases (RNases) play in the molecular underpinnings of Mendelian disorders. These enzymes, traditionally recognized for their involvement in RNA metabolism, have emerged as central actors whose dysfunction through genetic mutations can instigate a wide array of human diseases. The comprehensive analysis highlights how defects in RNase activity disrupt RNA dynamics, precipitating pathological conditions that span neurological impairments, growth anomalies, hematopoietic deficiencies, and mitochondrial dysfunctions.
Mutations that impair RNase function principally operate through loss-of-function mechanisms. These mutations often target the catalytic core domains of these enzymes or modify crucial motifs responsible for RNA recognition and subcellular localization. The resulting loss of enzymatic activity has been linked to a spectrum of severe phenotypes, including devastating conditions such as Aicardi-Goutières syndrome, amyotrophic lateral sclerosis, Perlman syndrome, and progressive external ophthalmoplegia. Notably, many of the RNases implicated in these diseases are evolutionarily conserved, underscoring their indispensable roles across biological systems.
The substrate specificity of RNases extends beyond messenger RNA, encompassing diverse classes of small non-coding RNAs including microRNAs (miRNAs) and PIWI-interacting RNAs (piRNAs). These small RNA molecules require precise RNase-mediated processing for their biogenesis and turnover. Disruption of RNase activity consequently perturbs RNA homeostasis, distorting gene regulatory networks essential for cellular function. In the context of neurological disease, such perturbations hinder asymmetric neuronal translation, interfere with innate immune surveillance, and impede the clearance of aberrant RNA species, ultimately fomenting neuroinflammation and synaptic failure.
.adsslot_YH2NWhxqpi{width:728px !important;height:90px !important;}
@media(max-width:1199px){ .adsslot_YH2NWhxqpi{width:468px !important;height:60px !important;}
}
@media(max-width:767px){ .adsslot_YH2NWhxqpi{width:320px !important;height:50px !important;}
}
ADVERTISEMENT
Beyond the nervous system, the role of RNases in regulating cellular signaling pathways is gaining increasing appreciation. Mutations in RNase genes can derail critical nodes in the PI3K/AKT/mTOR signaling pathway, instigating aberrant cell proliferation and organ overgrowth evident in certain congenital disorders. This mechanistic insight links fundamental RNA metabolism defects to systemic growth dysregulation, revealing a novel axis by which genetic lesions propagate phenotypic manifestations.
Hematopoiesis is another biological process vulnerable to RNase dysfunction. Mutations impair telomere maintenance and ribosome biogenesis, key processes underpinning hematopoietic stem cell renewal and differentiation. The resulting hematopoietic deficiencies manifest as anemia or immunodeficiency syndromes, reflecting the indispensable nature of RNase activity in maintaining blood cell homeostasis. These findings bridge molecular enzymology with clinical hematology, forging new paths for therapeutic intervention.
The review emphasizes the indispensability of model organisms to decode the complex genotype-to-phenotype relationships characteristic of RNase-linked diseases. Mouse models, zebrafish, Drosophila, Caenorhabditis elegans, and yeast serve as powerful platforms to dissect conserved genetic pathways and elucidate the cellular consequences of specific RNase mutations. Through these systems, researchers can examine perturbations in RNA stability, protein translation, and cellular stress responses with high resolution, facilitating the development of targeted therapies.
Recent advances in single-cell transcriptomics have accelerated the identification of RNase-related pathology by mapping cell-type-specific gene expression signatures and RNA metabolic changes. This technology, coupled with sophisticated cross-species genetic engineering tools, enables an unprecedented view into how RNase mutations alter cellular programs during development and disease progression. These insights are critical to tailoring personalized medicine strategies that address the molecular genesis of disorders at the level of individual cells.
Importantly, the review articulates the potential for therapeutic strategies targeting RNase pathways. Correcting or compensating for RNase deficiencies may restore normal RNA metabolism, attenuate cellular stress responses, and rescue impaired signaling axes. The integration of mechanistic studies with translational research holds promise for the development of novel interventions for diseases that have long eluded effective treatment due to their genetic complexity and pleiotropic manifestations.
This landmark synthesis of current knowledge also highlights the broader implications of RNase biology in human health. Beyond rare Mendelian disorders, RNase dysregulation may have bearings on cancer, immune dysfunction, and neurodegenerative diseases at large. Understanding the fundamental biology of these enzymes provides a conceptual framework that could revolutionize molecular medicine in the coming decades.
Despite the progress elucidated, several questions remain unresolved, such as the precise mechanisms by which specific RNase mutations translate to tissue-specific disease phenotypes. Moreover, the role of SLFN14, a ribonuclease excluded from this analysis due to incomplete characterization, represents an open frontier for future investigation. Continued research will be pivotal in uncovering the layers of regulation and interaction that govern RNA metabolism in health and disease.
In conclusion, this review consolidates ribonucleases as pivotal molecular custodians of RNA homeostasis, whose genetic aberrations underlie a spectrum of Mendelian diseases with complex clinical presentations. By leveraging model organism studies and cutting-edge transcriptomic technologies, the field is poised to transform our understanding of RNA metabolism into tangible diagnostic and therapeutic outcomes. This paradigm shift underscores the vital importance of RNases as both biomarkers and targets in the fight against genetically driven pathologies.
Subject of Research: Ribonucleases implicated in Mendelian diseases and their molecular mechanisms.
Article Title: Ribonucleases in Mendelian disease: Characterization and insight from model organisms.
News Publication Date: 2025.
Image Credits: Genes & Diseases.
Keywords: RNA metabolism, ribonucleases, Mendelian disorders, neurological disease, hematopoiesis, mitochondrial dysfunction, loss-of-function mutations, model organisms, RNA stability, PI3K/AKT/mTOR signaling, neuroinflammation, single-cell transcriptomics.
Tags: Aicardi-Goutières syndrome and RNasesevolutionary conservation of RNases in diseasesgenetic mutations affecting RNasesgrowth anomalies linked to RNase activityhematopoietic deficiencies and RNase functionMendelian disorders and RNA metabolismmitochondrial dysfunction and genetic mutationsneurological impairments and ribonucleasesribonucleases and genetic disordersRNase dysfunction in human diseasesRNase-mediated processing of microRNAs andsmall non-coding RNAs and ribonucleases