A groundbreaking editorial published in the latest edition of Aging-US reveals novel insights into the intertwined roles of polyploidy and cellular senescence in aging, tissue repair, and cancer development. The joint work of Iman M. Al-Naggar and George A. Kuchel from the University of Connecticut illuminates how polyploidy-induced senescence, a complex cellular state traditionally studied in isolation from other phenomena, may hold critical implications for understanding bladder biology and oncogenesis.
Cellular senescence, widely recognized as a stable arrest of cell division while maintaining metabolic activity, has traditionally been studied as a hallmark of aging and tumor suppression. Polyploidy, referring to cells harboring multiple genome copies beyond the standard diploid set, has a more ambiguous reputation. Frequently linked to cancer progression, polyploidy is paradoxically also a part of normal developmental processes and stress responses in healthy tissues. The editorial posits that these two biological phenomena — polyploidy and senescence — are not merely coincidental but may represent a coordinated cellular program essential for tissue homeostasis.
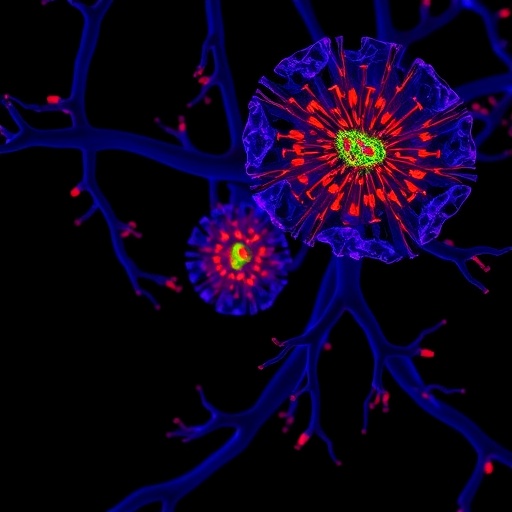
Central to the discussion are bladder umbrella cells, which form the critical urinary barrier separating urine from the bloodstream. In murine models, these cells undergo endoreplication during development, becoming polyploid and simultaneously exhibiting markers of senescence throughout the organism’s lifespan. This state is proposed to act less as a pathological condition and more as a robust differentiation mechanism that preserves tissue integrity. By stabilizing the bladder’s barrier function and sustaining resilience to environmental insults, polyploidy-induced senescence may support essential organ function.
The editorial further underscores the nuanced duality of polyploidy-induced senescence. While this cellular state confers protective advantages and aids in maintaining tissue architecture, it is dependent on intact checkpoint pathways including tumor suppressors like p16. If these pathways are compromised — whether through mutation, deletion, or epigenetic silencing — the senescent polyploid cells risk “depolyploidization.” This process involves erroneous re-entry into the cell cycle, which can enhance chromosomal instability, aneuploidy, and thus foster a microenvironment conducive to tumorigenesis.
The emerging connection between polyploidy-induced senescence and cancer initiation is particularly compelling in bladder cancers of urothelial origin, which are closely associated with aging. The authors hypothesize that a subset of aggressive bladder carcinomas may derive from polyploid umbrella cells that escape senescence barriers, highlighting a potential mechanistic link between aging-related cellular changes and oncogenic transformation. This offers a fresh perspective on tumor biology, deviating from traditional mutation-centric views to integrate altered cellular states shaped by polyploidy dynamics.
Beyond oncogenesis, the editorial touches upon implications for clinical oncology and therapeutic resistance. Many anticancer treatments paradoxically induce senescence and polyploidization in tumor cells as part of their mechanism to halt proliferation. However, this induced polyploidy can backfire: certain cancer cells may reduce their ploidy in a process called depolyploidization, resume division, and thereby contribute to disease relapse and treatment resistance. Understanding the biological interplay between polyploidy and senescence could thus inform the design of more effective therapeutic strategies that prevent such malignant adaptations.
The authors advocate for a paradigm shift in aging and cancer research to investigate polyploidy and senescence in concert. Current large-scale efforts mapping senescent cells across tissues seldom consider cellular ploidy, potentially overlooking a critical axis of biology. Including ploidy as a factor in senescence profiling could provide deeper insights into the aging process, tissue regeneration, and the early steps of tumor formation, ultimately guiding novel diagnostic and prognostic tools.
This editorial also integrates a developmental biology perspective, revealing that polyploidy-induced senescence is not restricted to the bladder but observed across multiple organs. In these contexts, polyploid senescence serves physiological functions, helping cells endure adverse microenvironments and maintain critical tissue-specific roles. Recognizing the evolutionary and functional conservation of this program broadens its relevance beyond pathology to foundational biology.
Crucial to the discussion is the molecular landscape underlying polyploidy-induced senescence. Markers such as senescence-associated β-galactosidase (SA β-gal) and phosphorylated histone variant γH2AX delineate the senescent state, often coupled with active DNA damage responses. The stability of this state depends heavily on tumor suppressor networks; disruption of these systems may unleash proliferative capacity in polyploid cells that otherwise act as a safety barrier against malignancy.
In summation, Al-Naggar and Kuchel’s editorial catalyzes a reevaluation of cellular senescence and polyploidy as not only hallmarks of aging but as intersecting programs with far-reaching implications in development, tissue homeostasis, and cancer. Their work encourages integrative research approaches that consider genomic content alongside cellular functional states, promising to enrich our understanding of biology and improve clinical outcomes in gerontology and oncology.
For further inquiries, the corresponding author Iman M. Al-Naggar can be contacted at [email protected]. The full editorial is accessible via Aging-US, DOI: 10.18632/aging.206355.
Subject of Research: Not applicable
Article Title: Polyploidy-induced senescence: Linking development, differentiation, repair, and (possibly) cancer?
News Publication Date: February 8, 2026
Web References:
Editorial: https://www.aging-us.com/article/206355/text
Journal site: http://www.aging-us.com/
DOI link: http://dx.doi.org/10.18632/aging.206355
References: Information taken directly from the editorial published in Aging-US
Image Credits: © 2026 Al-Naggar and Kuchel, Creative Commons Attribution License (CC BY 4.0)
Keywords: aging, cellular senescence, cancer, polyploidization, differentiation, urothelial carcinoma, oncogene-induced senescence
Tags: bladder umbrella cells polyploidycellular senescence and cancer riskcellular stress responses in agingendoreplication in tissue developmentmechanisms of tissue homeostasispolyploidy and metabolic activitypolyploidy and tumor suppressionpolyploidy effects on oncogenesispolyploidy in bladder biologypolyploidy-induced senescence in agingrole of polyploidy in tissue repairsenescence markers in polyploid cells