UNIVERSITY PARK, Pa. — In 2015, an outbreak of Zika virus, driven by a heavy rain season and subsequent boom in the virus’s host mosquito population, caused thousands of babies in Brazil to be born with severe birth defects. Zika virus is unique among flaviviruses, which also include West Nile, dengue and yellow fever viruses, in its ability to transmit from an infected mother to her unborn child.
Credit: Joyce Jose, Penn State
UNIVERSITY PARK, Pa. — In 2015, an outbreak of Zika virus, driven by a heavy rain season and subsequent boom in the virus’s host mosquito population, caused thousands of babies in Brazil to be born with severe birth defects. Zika virus is unique among flaviviruses, which also include West Nile, dengue and yellow fever viruses, in its ability to transmit from an infected mother to her unborn child.
How do the components of Zika virus assemble during viral replication and how does the virus then pass from mother to fetus? These are some of the questions that Joyce Jose, assistant professor of biochemistry and molecular biology at Penn State, and her colleagues aim to answer with two new grants from the U.S. National Institute of Allergy and Infectious Diseases totaling nearly $6 million.
“While human infections of Zika virus have declined since the devastating outbreak in 2015, the threat of future epidemics remains,” Jose said. “And with climate change, the mosquitoes that harbor Zika virus may shift their ranges northward, which could put even more people, including in the U.S., at greater risk, as well. Given that no vaccines or antiviral therapies currently exist, it is important to study the virus with a goal of informing the development of prevention measures.”
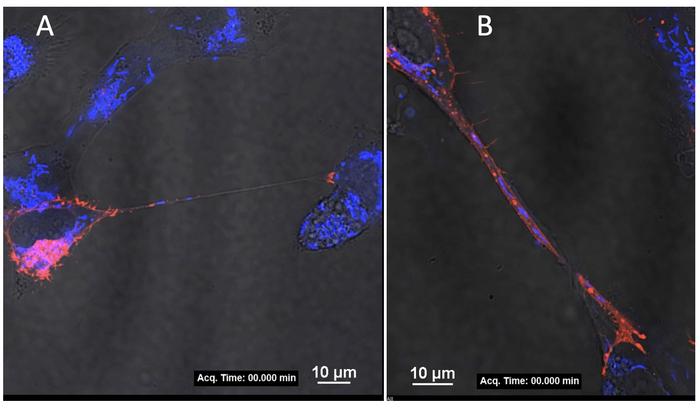
Jose explained that she and her colleagues recently discovered that maternal cells infected with Zika virus create connections, called tunneling nanotubes, that extend to uninfected cells, both within the mother and from mother to fetus. The findings of that study are currently under review and publicly available in preprint form.
“We think these tunneling nanotubes allow the virus to replicate in the mother and pass from mother to baby,” Jose said. “In addition to providing a mechanism to cross the placenta, these nanotubes may enable the virus to avoid the host’s immune response. We call this a stealth mode of transmission.”
In their previous research, the team found that a certain protein, called non-structural protein 1 (NS1), may be responsible for inducing the formation of these nanotubes. With one of the new grants, totaling nearly $4 million, Jose and her colleague Indira Mysorekar, professor of medicine at Baylor College of Medicine, will conduct experiments in human cells in vitro, meaning in a culture dish, and in vivo, meaning in live mice, to examine the specific regions of NS1 that may participate in signaling or interacting with proteins on human cells to initiate the formation of the nanotubes.
“Discovering which regions of NS1 are ‘talking’ to human proteins is the first step in understanding how nanotube formation begins,” Jose said. “If we can figure out how this protein is working, we can potentially target the protein with drugs that disable its function. Interestingly, when I was a postdoc, my lab was instrumental in solving the structure of dengue virus NS1, so now I’ve gone back to my roots to further study this important protein.”
Next, the researchers will study whether whole virus particles or just viral RNA, or genetic material, are transported through the nanotubes.
“In our previous research, we documented components of the virus, like RNA and certain proteins, inside these nanotubes, so the question is whether the whole virus or just components of the virus can pass through,” Jose said.
Additionally, the researchers plan to further investigate the role of tunneling nanotube formation in the transmission of Zika virus from mother to fetus by examining the virus in action in pregnant mice. Finally, the team will study mitochondria transport through these nanotubes.
“In addition to viral components, we have observed host cell organelles, including mitochondria, in these nanotubes,” Jose said. “Mitochondria are the cell’s powerhouses, so by hijacking these energy-generating organelles, the virus could be fueling its own ability to infect cells.”
Jose noted that other viruses, including HIV, are known to produce similar nanotubes.
“We have drugs that inhibit nanotube formation for HIV, so if we can develop something similar for Zika virus, we may be able to develop therapeutic interventions to prevent pregnant women who are infected with the virus from transmitting it to their baby,” Jose said.
So far, Jose said that other flaviviruses do not appear to have the ability to produce tunneling nanotubes, with the exception of West Nile virus to a small degree.
“A question we have is whether other flaviviruses could evolve this ability at some point,” Jose said. “In addition to helping to avoid the devastating consequences of another Zika virus outbreak, our research could help in monitoring other flaviviruses that may evolve the ability to form nanotubes.”
Jose and her colleagues are also studying mechanisms by which Zika virus assembles in host cells prior to inducing tunneling nanotubes. In previous research that published recently in the journal npj Viruses and PLOS Neglected Tropical Diseases, they specifically investigated processes within Zika virus that prime it for infection of human cells.
“In one study, we discovered a ‘latch and lock’ mechanism by which two Zika virus proteins connect to stabilize and prepare the virus to infect human cells,” Jose said. “In the other, we found an interaction between Zika virus’s capsid protein and viral membrane protein that helps us to understand how viruses assemble within host cells during their replication.
With a second NIH grant, totaling nearly $2 million, Jose will also examine additional non-structural proteins (NS2 and NS4) to investigate their role in viral assembly.
“Our work will document for the first time the feasibility of a powerful live imaging approach to study the trafficking of viral components, including RNA and proteins, as well as the virus potentially co-opting host factors to infect human cells,” Jose said.
Jose explained that understanding all the phases of Zika virus’s infection, replication and transmission may aid in identifying potential targets for therapeutics.
“These studies,” she said, “may help us to understand, treat and prevent flavivirus infections and address a major global public health need.”




