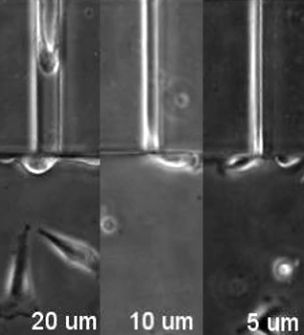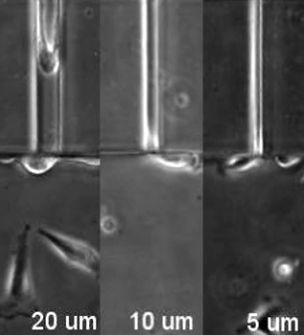
As cells move throughout our bodies, they often have to squeeze through tight nooks and crannies in their environment, reliably springing back to their original shape. The structures involved in this process are still a mystery, but in a study published August 22 in Developmental Cell, a research team reports one protein responsible for giving a cell’s nucleus its durable, deformable nature. These results, the authors say, may explain the invasiveness of certain cancer cells.
“The nucleus is the fattest organelle in the whole cell, and really needs to be squished if a cancer cell wants to metastasize throughout the body,” says Maddy Parsons, Professor of Cell Biology at King’s College London, whose research group led the study. “There’s a really complex network of proteins that keeps the cell from buckling in on itself when it’s faced with a tight squeeze.”
The researchers pinpointed one protein responsible–fascin–that is known to be at unusually high levels in cancer cells. Originally, says Parsons, fascin was only thought to exist at the cell’s edge as a “bundling protein,” where it binds and stabilizes spiky finger-like protrusions called filopodia at the cell’s plasma membrane. These structures allow the cell to sense its surrounding environment and pull itself through tissue. Now, the researchers have found that fascin also sits at the periphery of the nuclear envelope (the membrane that surrounds the nucleus), binding to different structures there to add stability and prevent collapse.
“All cells have a cytoskeleton made from lots of filaments of a protein called actin that give the cell its architecture, but without other proteins added on, the skeleton will buckle under stress,” says Parsons. “Proteins like fascin that bind onto this cytoskeleton add an element of stability and flexibility–it makes the cell more mechanically stable and able to rapidly respond to the environment to change shape. This data we have, showing fascin at the nuclear periphery, has opened up a new way of thinking about how this protein controls cancer cell movement.”
To investigate how fascin controls cancer cell migration, Parsons and her team in one set of experiments placed human cancer cells into a series of artificial microchannels, which are small devices with pore sizes ranging from large pores that a cell can easily maneuver through to spaces as small as 2 microns wide, into which the cell can barely fit. By reducing intercellular levels of the fascin protein, they found that the cells were unable to squish their nuclei enough to fit into small channels. This suggests that cancer cells may use fascin to better navigate through different densities of tissues that they face during their invasion away from a solid tumor.
“I think what we’re showing is that fascin has more than one important role in controlling cancer cell metastasis,” Parsons adds. “Importantly, this is also revealing new ways that we can target fascin to block its function in cancer cells.”
Parsons and her team are still eager to determine the forces controlling fascin in cancer cells. “We’re really still in the dark about what proteins are controlling these different functions of fascin,” she says. “We still don’t know what proteins and signals are telling fascin to move from the cell’s edge to the nucleus, so these are the next questions we hope to tackle.”
###
The authors were funded by the Medical Research Council, the Royal Society, and the Wellcome Trust.
Developmental Cell, Jayo et al.: “Fascin regulates nuclear movement and deformation in migrating cells,” http://www.cell.com/developmental-cell/fulltext/S1534-5807(16)30514-7
Developmental Cell (@Dev_Cell), published by Cell Press, is a bimonthly, cross-disciplinary journal that brings together the fields of cell biology and developmental biology. Articles provide new biological insight of cell proliferation, intracellular targeting, cell polarity, membrane traffic, cell migration, stem cell biology, chromatin regulation and function, differentiation, morphogenesis and biomechanics, and regeneration and cellular homeostasis. Visit: http://www.cell.com/developmental-cell. To receive Cell Press media alerts, contact [email protected].
Media Contact
Shoshana Wodinsky
[email protected]
617-397-2802
@CellPressNews
http://www.cellpress.com
The post How cell nuclei squeeze into tight spaces appeared first on Scienmag.