In the rapidly evolving landscape of cellular biology, researchers continue to uncover intricate mechanisms that govern cellular function and immune regulation. A groundbreaking study recently published in Cell Death Discovery sheds new light on the pivotal role of the chaperone protein GRP94 in regulating the maturation of transforming growth factor-beta (TGF-beta) within human primary M2 macrophages. This discovery not only advances our understanding of macrophage biology but also opens new avenues for therapeutic intervention in diseases characterized by immune dysregulation and fibrosis.
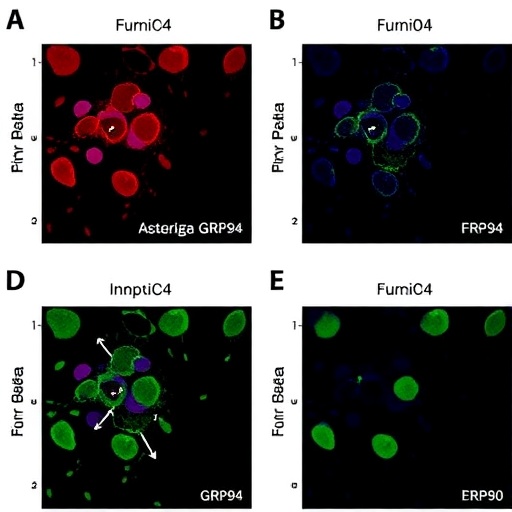
Central to this research is the endoplasmic reticulum-resident chaperone protein GRP94, a member of the HSP90 family, known for its critical involvement in protein folding and quality control. GRP94’s client proteins include a spectrum of receptor molecules and secretory proteins crucial for cell signaling and homeostasis. This study uncovers that GRP94 engages in a direct and functional interaction with the proprotein convertase furin, a pivotal protease responsible for processing numerous substrates, including growth factors, receptors, and viral proteins. The intimate crosstalk between GRP94 and furin uncovered here suggests a carefully orchestrated mechanism whereby GRP94 regulates the bioactivation of key signaling molecules.
M2 macrophages, alternatively activated macrophages characterized by anti-inflammatory and tissue repair properties, are central effectors in immune modulation. The study reveals that in these cells, GRP94’s interaction with furin is instrumental in controlling the maturation of latent TGF-beta precursors. TGF-beta, a multifunctional cytokine, requires precise proteolytic cleavage to release its active form, crucial for mediating cellular differentiation, proliferation, and extracellular matrix production. The findings indicate that GRP94 ensures the correct conformational and functional presentation of furin, thereby fine-tuning the cleavage and activation of TGF-beta within the cellular milieu.
Delving deeper, the researchers demonstrate that disrupting GRP94 function leads to a significant reduction in furin’s proteolytic activity, culminating in a downstream inhibition of TGF-beta maturation. This effect highlights a previously unrecognized regulatory axis whereby chaperone-mediated control at the level of protein folding and enzyme activation directly influences cytokine availability and function. Such mechanistic insights provide a molecular framework explaining how macrophages calibrate their immune responses through modulating extracellular signaling cascades.
Methodologically, the study harnesses an impressive repertoire of cutting-edge techniques, including co-immunoprecipitation assays to elucidate protein-protein interactions, enzyme activity assays to quantify furin function, and flow cytometry for assessing macrophage phenotypes. Confocal microscopy further establishes the subcellular colocalization of GRP94 and furin within the endoplasmic reticulum, reinforcing the spatial context of their interaction. These complementary approaches combine to create an in-depth and multi-dimensional perspective on how molecular chaperones orchestrate proprotein convertase activity in immune cells.
From a broader perspective, the implications of this regulatory mechanism extend beyond basic biology, intersecting with pathophysiological conditions marked by aberrant TGF-beta signaling. Fibrotic diseases, cancer progression, and chronic inflammatory states often hinge upon dysregulated activation of growth factors like TGF-beta. By delineating how GRP94 modulates furin-dependent TGF-beta maturation, this study paves the way for novel therapeutic strategies aimed at targeting the molecular chaperone machinery to mitigate excessive or inappropriate TGF-beta activation.
Moreover, these findings challenge existing paradigms, which typically consider proprotein convertases as autonomous enzymes with intrinsic regulatory controls. The newly identified dependency on GRP94 adds a layer of complexity and nuance to our understanding of protease regulation, suggesting that chaperone systems can exert upstream control over proteolytic cascades. This could have significant ramifications for the development of pharmacological agents targeting chaperones, traditionally pursued in oncology and neurodegeneration.
Intriguingly, the study also hints at potential crosstalk between stress pathways and immune function. Given that GRP94 is a stress-inducible chaperone elevated during endoplasmic reticulum stress, its role in modulating furin and consequently TGF-beta maturation may link cellular stress responses with immune modulation. This intersection offers fertile ground for exploring stress-associated diseases and devising interventions that address both protein homeostasis and immune regulation concurrently.
Importantly, the focus on human primary M2 macrophages underscores the physiological relevance of these findings. Unlike immortalized cell lines, primary cells preserve native differentiation states and functional profiles, enhancing the translational value of the research. This also reflects the heterogeneity status of macrophage populations in vivo, where the balance between pro-inflammatory M1 and anti-inflammatory M2 subsets critically affects disease outcomes.
Another fascinating aspect is the potential feedback loops implied by this interaction. Activated TGF-beta itself modulates immune cell differentiation and function, possibly affecting GRP94 expression or activity, which in turn impacts furin efficiency. Such feedback could establish regulatory circuits ensuring homeostasis or fueling pathological states when disrupted. Future research elucidating these dynamic relationships might uncover additional layers of immune regulation.
This pioneering study exemplifies the power of integrating molecular biology with immunology to unravel complex cellular processes. Understanding how chaperone proteins like GRP94 exert precise control over essential enzymatic functions reveals the exquisite regulatory networks that underpin immune responses. Such insights not only deepen our grasp of cell biology but also sharpen the tools for designing targeted interventions that can fine-tune immune activity in health and disease.
The potential for GRP94 as a therapeutic target is particularly exciting in light of this research. Pharmacological modulation of chaperone activity to influence furin function and TGF-beta maturation could offer innovative treatments for fibrotic disorders, autoimmune diseases, and cancer. Such strategies might harness small-molecule inhibitors or stabilizers of GRP94, providing nuanced control over cytokine signaling without complete ablation of essential protease functions.
Furthermore, these findings underscore the importance of proteostasis networks in immune cell functionality. By linking chaperone systems directly to cytokine maturation pathways, this study expands our appreciation of how protein folding, enzyme activation, and receptor signaling converge to sculpt immune landscapes. Understanding these intersections is crucial for developing comprehensive models of immune regulation and dysfunction.
In conclusion, the discovery that GRP94 interacts with and regulates the proprotein convertase furin, thereby controlling TGF-beta maturation within M2 macrophages, represents a significant advancement in cellular immunology. This research elucidates a novel chaperone-protease axis that fine-tunes cytokine availability and immune phenotype modulation. As we continue to decode these intricate mechanisms, the opportunities for translating such knowledge into clinical applications promise to transform therapeutic approaches to a host of inflammatory and fibrotic diseases, marking an exciting frontier in biomedical science.
Subject of Research: The interaction between the chaperone protein GRP94 and the proprotein convertase furin, with an emphasis on the regulation of TGF-beta maturation in human primary M2 macrophages.
Article Title: The chaperone GRP94 interacts with the proprotein convertase furin and regulates TGF-beta maturation in human primary M2 macrophages.
Article References:
Baverel, V., Wang, F., Garrido, C. et al. The chaperone GRP94 interacts with the proprotein convertase furin and regulates TGF-beta maturation in human primary M2 macrophages. Cell Death Discov. 11, 558 (2025). https://doi.org/10.1038/s41420-025-02866-2
Image Credits: AI Generated
DOI: 15 December 2025
Tags: cellular signaling pathwaysendoplasmic reticulum functionfurin protease interactionGRP94 chaperone proteinHSP90 family proteinsimmune dysregulation interventionsimmune modulation mechanismsmacrophage biology researchprimary M2 macrophages roleprotein folding quality controlTGF-beta maturation regulationtherapeutic targets in fibrosis