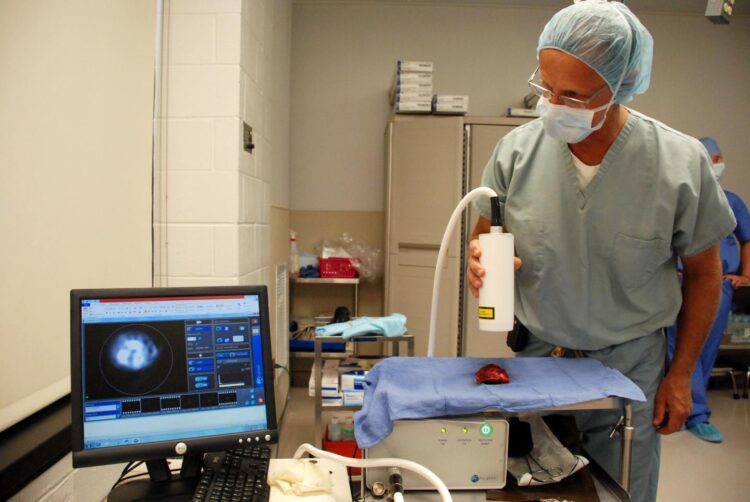
In dogs with mammary tumors, researchers from the University of Pennsylvania used a substance that glows under near-infrared light to illuminate cancer
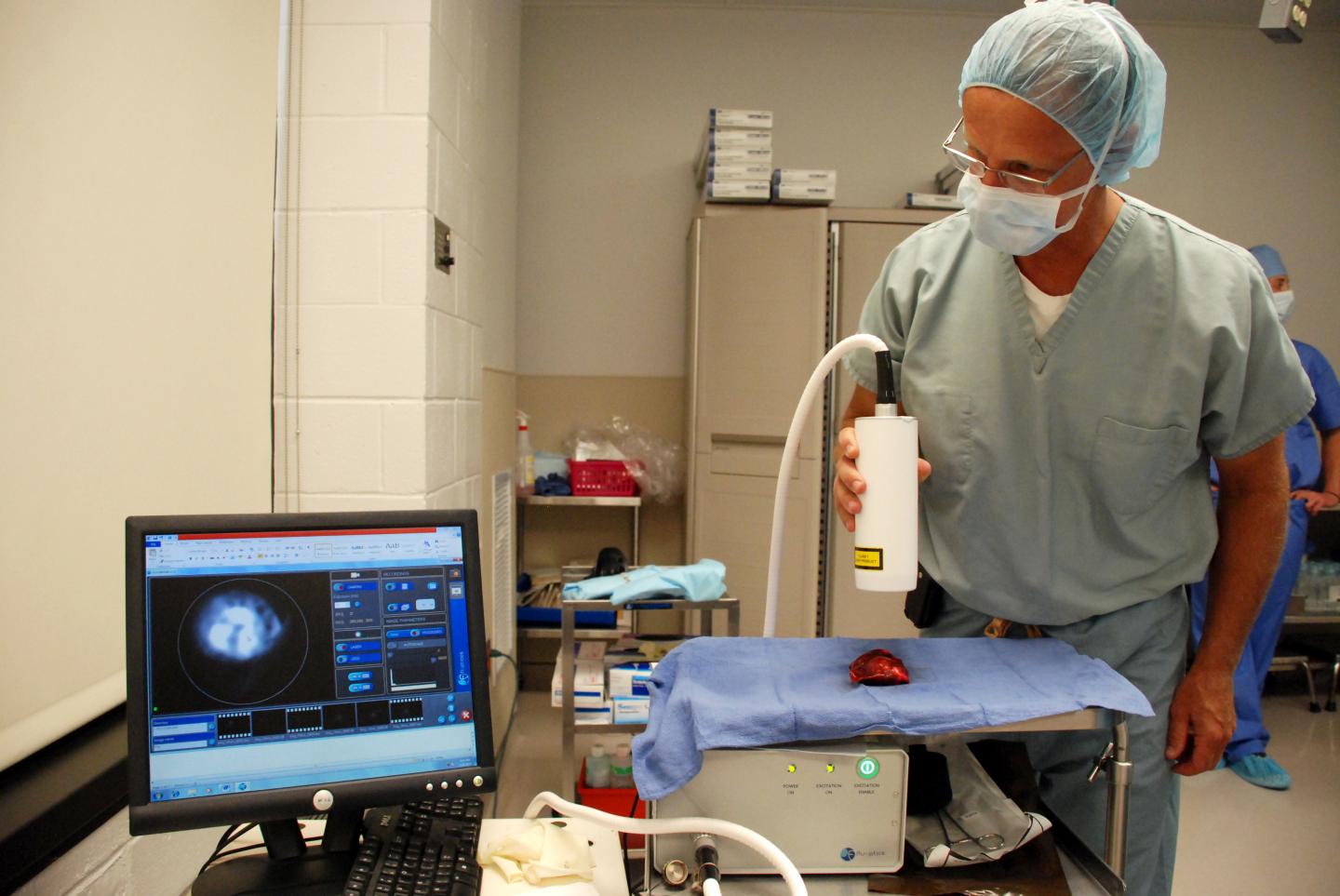
Credit: John Donges/School of Veterinary Medicine
“Clean margins” are a goal of cancer excision surgery. If even a small piece of cancerous tissue is left behind, it increases the likelihood of a local recurrence and spread of the disease, possibly reducing overall survival time.
With an innovative approach to cancer surgery, researchers at the University of Pennsylvania are investigating a technique to help surgeons clearly see whether they’ve left any diseased tissue behind. Using a dye that glows under near-infrared light and preferentially accumulates in cancer cells, they performed surgery to remove mammary tumors from dogs treated at the School of Veterinary Medicine’s Ryan Hospital.
They found that the technique was able to illuminate not only the tumors but also cancer that had spread to the lymph nodes. Mammary cancer in dogs is akin to human breast cancer in many key ways. The research team believes that, with a different dye that is more specifically targeted to cancer cells, a parallel technique could improve outcomes in breast cancer patients who opt for breast-conserving surgery to treat their disease, The researchers reported this in the journal PLOS ONE.
“Doing this kind of research has two main benefits,” says David Holt, a veterinary surgeon and senior author on the work. “The dogs are a great model for human breast cancer, but there are also some real opportunities to benefit the dogs as well.”
A team from Perelman School of Medicine led by Sunil Singhal of the Center for Precision Surgery at the Abramson Cancer Center in collaboration with Holt and others at Penn Vet have been using the FDA-approved contrast agent indocyanine green (ICG), which glows under near-infrared light, to attempt to differentiate normal from cancerous tissue for several years in different types of cancer. Scientists believe ICG accumulates in cancer because it leaks out through the fast-growing blood vessels in tumors, which tend to be more permeable than normal vessels in healthy tissue.
The aim of the current work was to test the technique in pet dogs with mammary tumors as a model for breast conserving surgery in women. All pet owners gave consent to be part of the study. The day before surgery, dogs received an injection of ICG. The surgeries themselves, either lumpectomies or mastectomies, proceeded as they normally would, following standard-of-care procedures. Then, under near-infrared light, the surgeons observed the excised tumors as well as the surgical site to look for signs of glowing ICG.
In dogs, since aesthetics are less of a concern, surgeons generally take much wider margins when excising mammary tumors than is done when performing breast-conserving surgery on a person. So, the study wasn’t able to detect remnant “dirty edges” after excision. They did, however, find larger tumors accumulated more dye.
The research team was also interested in looking at the dogs’ lymph nodes.
“In women with breast cancer and also in dogs with mammary cancer,” Holt says, “it’s prognostic if the cancer has spread to the lymph nodes. What we showed was that we could identify both draining lymph nodes and lymph nodes with metastatic disease.”
Currently in human medicine, radioisotopes administered into the breast are often used to identify draining or “sentinel” lymph nodes. Using a near-infrared imaging agent administered intravenously means that radioactive agent and the protective shielding that it necessitates are not required.
“If we could give an injection before the surgery that would identify just the lymph nodes that are potentially problematic, it would avoid a lot of the risk of either removing too many lymph nodes or leaving in those that are have metastatic disease,” says Holt.
In concurrent and follow-up work, Holt and his counterparts at Penn Medicine are continuing to investigate the efficacy of using targeted near-infrared imaging agents in cancer patients. These dyes bind more specifically to cancer cells, helping better define “clean margins” for both human and canine cancer patients.
###
Holt’s coauthors on the work were Andrew Newton, Jarrod Predina, and Sunil Singhal of the Perelman School of Medicine and Michael Mison, Jeffrey Runge, Charles Bradley, and Darko Stefanovski of the School of Veterinary Medicine.
The work was supported by the Mari Lowe Center for Comparative Oncology at the University of Pennsylvania School of Veterinary Medicine.
Media Contact
Katherine Unger Baillie
[email protected]
Original Source
https:/
Related Journal Article
http://dx.