In Alzheimer’s disease, plaques of amyloid beta protein accumulate in the brain, damaging connections between neurons. Now, researchers at University of California San Diego School of Medicine and Harvard Medical School have found that the enzyme Protein Kinase C (PKC) alpha is necessary for amyloid beta to damage neuronal connections. They also identified genetic variations that enhance PKC alpha activity in patients with Alzheimer’s disease.
The study, published May 10 in Science Signaling, may present a new therapeutic target for the disease.
“Until recently, it was thought that PKC helped cells survive, and that too much PKC activity led to cancer. Based on that assumption, many companies tested PKC inhibitors as drugs to treat cancer, but they didn’t work,” said co-senior author Alexandra Newton, PhD, professor of pharmacology at UC San Diego School of Medicine.
“Instead, we recently found that the opposite is true. PKC serves as the brakes to cell growth and survival, so cancer cells benefit when PKC is inactivated. Now, our latest study reveals that too much PKC activity is also bad, driving neurodegeneration. This means that drugs that failed in clinical trials for cancer may provide a new therapeutic opportunity for Alzheimer’s disease.”
The study was a three-way collaboration between experts in PKC (Newton), neuroscience (Roberto Malinow, MD, PhD, Distinguished Professor of Neurosciences and Neurobiology at UC San Diego School of Medicine), and genomics (Rudolph Tanzi, PhD, professor of neurology at Harvard Medical School).
Malinow’s team found that when mice are missing the PKC alpha gene, neurons functioned normally, even when amyloid beta was present. Then, when they restored PKC alpha, amyloid beta once again impaired neuronal function. In other words, amyloid beta doesn’t inhibit brain function unless PKC alpha is active.
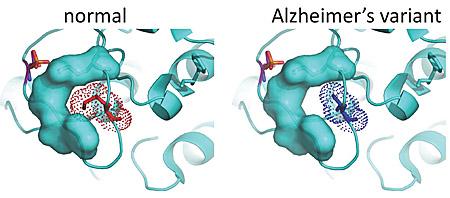
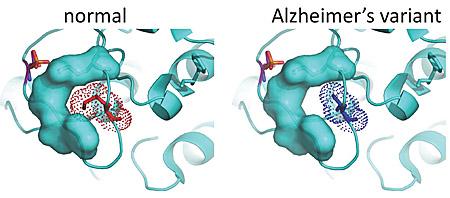
Enter the Tanzi team, which has a database of genetic information for 1,345 people in 410 families with late-onset Alzheimer’s disease. Tanzi and team use this database to look for rare variants — genetic mutations found only in family members with the disease. Here, the team found three variants in one form of the PKC enzyme, PKC alpha that were associated with the disease in five families.
The researchers replicated these three PKC alpha gene variants in laboratory cell lines. In each instance, PKC alpha activity was increased.
While this study surfaced only five families with these rare mutations in the PKC alpha gene, there are many ways to influence PKC alpha’s activity, Newton said. She believes there could be many other inherited genetic variations that indirectly boost or inhibit PKC activity, and therefore also influence a person’s likelihood of developing Alzheimer’s disease.
“Next we want to identify more molecules participating in the pathophysiology,” said Malinow. “The more steps in the mechanism we can understand, the more therapeutic targets we’ll find for Alzheimer’s disease.”
###
Co-authors of this study also include: Stephanie Alfonso, Julia A. Callender, Corina E. Antal, UC San Diego; Basavaraj Hooli, Kristina Mullin, Harvard Medical School; Mathew A. Sherman, Sylvain E. Lesné, University of Minnesota; and Michael Leitges, University of Oslo.
This research was funded, in part, by the National Institutes of Health (grants GM43154, MH060009, AG032132, GM007752, DGE1144086) and Cure Alzheimer’s Fund (grant 2015-3999).
Media Contact
Heather Buschman
[email protected]
619-543-6163
@UCSanDiego
http://www.ucsd.edu
The post Genetic variations that boost PKC enzyme contribute to Alzheimer’s disease appeared first on Scienmag.