In the dynamic landscape of cellular biology, oxygen availability stands as a fundamental determinant of cell fate, survival, and function. Oxygen deprivation, or hypoxia, is a hallmark of numerous physiological and pathological states, including cancer progression, ischemic tissue injury, and stem cell maintenance. While the hypoxia-inducible factor 1 alpha (HIF-1α) protein has long been recognized as the master regulator orchestrating cellular adaptation to low oxygen environments, the nuanced molecular mechanisms by which cells decode variable oxygen levels and fine-tune HIF-1α activity have remained elusive. A recent breakthrough study led by Wei Wang and colleagues at Nanjing University unveils a sophisticated multi-tiered regulatory framework that controls HIF-1α activation in response to graded hypoxia, shedding light on an intricate system of enzymatic modulation and feedback dynamics.
HIF-1α functions as a pivotal transcription factor that governs an array of genes facilitating metabolic recalibration, immune modulation, angiogenesis, and survival pathways under hypoxic stress. The core of this regulatory control involves oxygen-dependent hydroxylation catalyzed by two classes of enzymes: prolyl hydroxylases (PHDs) and factor inhibiting HIF (FIH). Under normoxic conditions, these hydroxylases mark HIF-1α for proteasomal degradation and suppress its transcriptional activation domains. However, the precise sequence and gradation through which these hydroxylases are deactivated as oxygen levels decline were poorly understood until now.
Employing an integrative approach combining mathematical modeling, dynamic simulation, bifurcation analysis, and rigorous experimental validation, Wang’s team constructed a quantitative regulatory network that captures the stepwise activation of HIF-1α under diminishing oxygen tensions. Their model elegantly demonstrates that HIF-1α stabilization and activation do not occur as a binary switch but rather progress in discrete stages dictated by the differential sensitivity of PHDs and FIH to oxygen. This refined understanding illuminates how cells interpret the subtle hypoxic continuum—from mild to severe hypoxia—and mount graded adaptive responses accordingly.
.adsslot_hFVuXyEwom{width:728px !important;height:90px !important;}
@media(max-width:1199px){ .adsslot_hFVuXyEwom{width:468px !important;height:60px !important;}
}
@media(max-width:767px){ .adsslot_hFVuXyEwom{width:320px !important;height:50px !important;}
}
ADVERTISEMENT
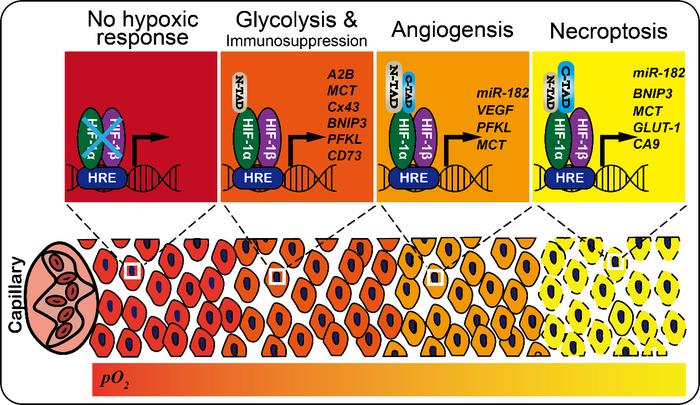
At the initial phase of mild hypoxia, approximately 2% oxygen, prolyl hydroxylases are selectively inhibited. This inhibition results in the accumulation of HIF-1α protein by preventing its degradation and exposes the N-terminal transactivation domain (N-TAD). The partial activation conferred by N-TAD leads to transcriptional upregulation of genes primarily involved in glycolytic metabolism enhancement and immune response suppression. These early adaptations allow cells to optimize energy production and modulate their microenvironment in response to modest oxygen shortages.
As oxygen tension further decreases to moderate levels near 0.7%, factor inhibiting HIF (FIH) activity is also impeded. With FIH inactivation, the C-terminal transactivation domain (C-TAD) of HIF-1α becomes accessible, culminating in full transcriptional activation. This stage is characterized by robust induction of angiogenic factors, promoting neovascularization to restore oxygen supply. The activation of angiogenesis at this hypoxia tier represents a strategic cellular investment in long-term survival, facilitating tissue remodeling and vascular adaptation.
Under conditions of severe hypoxia, defined as oxygen concentrations below 0.5%, HIF-1α is fully stabilized and activated at maximal levels. This state triggers a crescendo of downstream effects, including accumulation of lactate due to persistent anaerobic glycolysis, acidification of the cellular microenvironment, and ultimately, the initiation of programmed necrosis pathways. This terminal response highlights the cell’s shift from adaptation toward sacrificial processes when oxygen deprivation becomes untenable.
Intriguingly, the study identifies microRNA-182 (miR-182) as a dynamic modulator that fine-tunes HIF-1α’s transcriptional output throughout these activation stages. Acting as a “sliding regulator,” miR-182 dynamically modulates the feedback loops involving HIF-1α, PHD-2, and FIH to sharpen the sensitivity and precision of oxygen sensing. This dual feedback architecture balances positive amplification and negative regulation, enabling cells to respond to fluctuating oxygen levels with remarkable fidelity and adaptability.
The implications of this tiered hydroxylase deactivation and HIF-1α activation model are profound. By unveiling distinct transcriptional configurations tied to precise hypoxic thresholds, the work provides a mechanistic blueprint for “precision targeting” within the HIF signaling cascade. Therapeutically, this opens avenues for designing phase-specific interventions, such as selective inhibitors targeting glycolytic enzymes during mild hypoxia or anti-angiogenic agents tailored for moderate hypoxic zones, enhancing treatment specificity and efficacy.
Moreover, the advances in oxygen-sensing technologies, including spatially resolved probes capable of mapping oxygen gradients deep within tissues such as bone marrow, offer exciting opportunities to apply these insights in vivo. Understanding the spatial hypoxic heterogeneity within tumors, and how different hypoxic niches activate unique HIF-1α transcriptional programs, can inform better stratification of therapeutic modalities. Such spatial hypoxia profiling may elucidate why certain regions of tumors exhibit differential drug resistance or support immune evasion, providing critical guidance for treatment planning.
The research further highlights the importance of integrating hypoxia compartmentalization into multimodal therapy design. By accounting for the interplay between drug diffusion limitations and distinct hypoxia-driven cellular adaptations, combination regimens can be optimized to circumvent compensatory resistance mechanisms. This integrated approach holds promise to transform current monotherapies, which often fail due to incomplete hypoxia targeting, into more robust, synergistic strategies capable of effectively disrupting tumor growth and progression.
Future exploration will undoubtedly focus on expanding this regulatory network to include additional noncoding RNAs, post-translational modifications, and metabolic feedbacks that intersect with the HIF pathway. Furthermore, dissecting how cyclic and dynamic hypoxia—rather than static low oxygen—modifies HIF-1α activation patterns remains a fertile area of investigation. These refinements will consolidate our molecular understanding of cellular oxygen sensing and adaptation, accelerating the development of hypoxia-based precision medicine.
In sum, this seminal study reframes the classical view of hypoxia adaptation from a simple oxygen sensor narrative to a complex, layered regulatory choreography driven by progressive hydroxylase deactivation and sophisticated feedback control. It offers both a conceptual and practical framework to decode cellular oxygen responses, paving the way for innovative diagnostic and therapeutic advancements in diseases characterized by hypoxic stress.
Subject of Research: Cellular adaptation to graded hypoxia mediated by HIF-1α regulatory networks.
Article Title: Progressive Deactivation of Hydroxylases Controls Hypoxia-Inducible Factor-1α-Coordinated Cellular Adaptation to Graded Hypoxia
News Publication Date: 1-Apr-2025
Web References: http://dx.doi.org/10.34133/research.0651
Image Credits: Copyright © 2025 Ping Wang et al.
Keywords
Hypoxia, HIF-1α, prolyl hydroxylases, factor inhibiting HIF, oxygen sensing, cell fate, angiogenesis, metabolic reprogramming, microRNA-182, tumor microenvironment, graded hypoxia, feedback regulation
Tags: angiogenesis and hypoxiacellular response to oxygen deprivationenzymatic modulation of HIF-1αfeedback dynamics in hypoxic environmentsHIF-1α regulation in hypoxiahypoxia-inducible factors in cancer progressionischemic tissue injury and HIF-1αmechanisms of hypoxia in cancermetabolic adaptation in cancer cellsprolyl hydroxylases role in HIF-1αtranscription factors in cellular adaptationtumor cell survival under low oxygen