Credit: IST Austria
A simple ball of cells is the starting point for humans — and zebrafish. At the end of embryonic development, however, a fish and a human look very different. The biochemical signals at play have been studied extensively. How mechanical forces on the other hand shape the embryo is the subject of a study by Carl-Philipp Heisenberg, Professor at the Institute of Science and Technology Austria (IST Austria), and his group, including first author and postdoc Michael Smutny. In their study, published today in Nature Cell Biology, the researchers show that friction between moving tissues generates force. This force shapes the nervous system of the zebrafish embryo, a popular animal model of embryonic development. "We show that friction is generated by forming tissues sliding against each other, and that this force is a key mechanism for regulating morphogenesis during embryo development," Carl-Philipp Heisenberg explains.
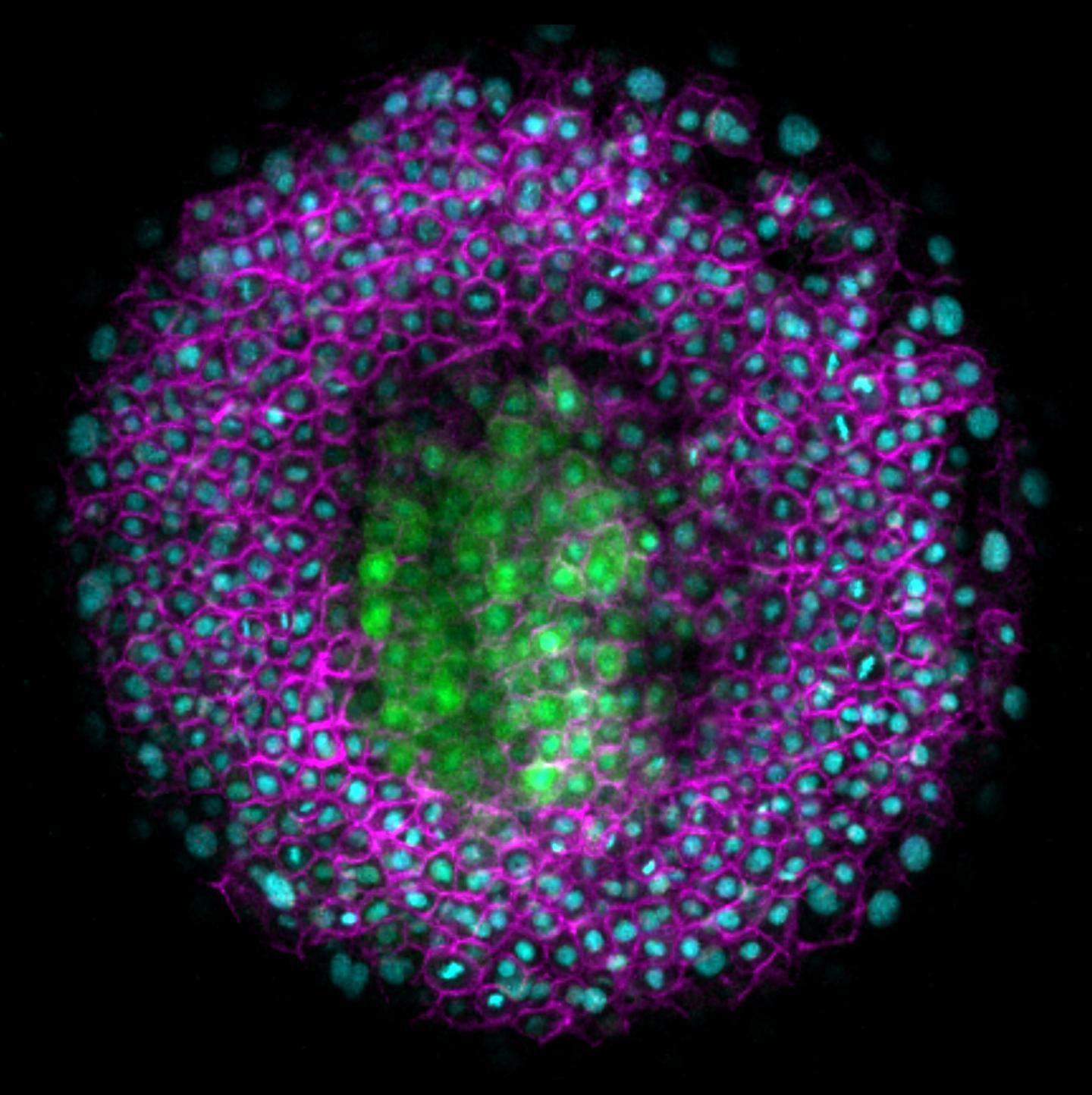
As the embryo develops, cells move and tissues are rearranged. Mechanical forces drive this morphogenesis. So far, however, it has been poorly understood how these forces are generated and integrated with other signals. In the present study, Heisenberg and his group studied the mechanical forces that are at work when the central nervous system (CNS) of the zebrafish develops. The neural anlage, the precursor of the neural tube, develops from one of three germ layers, the neurectoderm. However, the other two germ layers, the mesoderm and endoderm, have been shown to be crucial for the proper morphogenesis of the neurectoderm. When the neural anlage develops, the germ layers of the ball-shaped embryo move in opposite directions. The mesoderm and endoderm — also referred to collectively as mesendoderm – move to one pole of the embryo, the so-called animal pole, while the overlying neurectoderm slides against them to move to the opposite pole, the vegetal pole.
Heisenberg and his group found that this movement is important for positioning the neural anlage correctly. As the tissues slide against each other, the cells in the neurectoderm that will form the neural anlage change their direction of movement. They switch track and move towards the animal pole, the same direction as the underlying mesendoderm. The researchers found that in embryos where the mesendoderm is absent, these neurectoderm cells do not reorient. Instead, all neurectoderm cells move to the vegetal pole and the neural anlage is incorrectly positioned. When the mesendoderm cells move more slowly than normal, the neural anlage also ends up at the wrong position.
Now, to find what the underlying mechanism was, the researchers built a theoretical model based on their observation. By modelling the forces at work in the embryo, they found that the movement of neurectoderm against mesendoderm causes friction to arise. Michael Smutny explains how friction arises: "When the tissues slide against each other, friction arises, similar to when you rub a balloon against a sweater. In the case of the zebrafish embryo, the tissues contact each other directly via E-cadherin, a protein that reaches out of the cells. When these linker proteins rub against each other, friction builds up between the tissues."
The scientists confirmed the importance of E-cadherin by rebuilding the system in the lab: they cultured a layer of ectoderm cells in a dish and moved it in one direction, while pushing a bead coated with E-cadherin in the opposite direction. As a result, the ectoderm cells re-orient in the same way as observed in the embryo. This finding that mesendoderm cells directly affect the movement of neurectoderm cells through friction forces shows for the first time that friction is a key regulator of tissue morphogenesis in the embryo.
Neurectoderm morphogenesis defects are one of the most common birth defects in humans. The finding that friction forces that emerge at the interface between the forming germ layers play a key role in neurectoderm morphogenesis indicate a previously unrecognized mechanism that might underlie those birth defects.
###
Media Contact
Elisabeth Guggenberger
[email protected]
43-224-390-001-199
@istaustria
http://Www.ist.ac.at
############
Story Source: Materials provided by Scienmag