Credit: MBI
A collaborative ultrafast spectroscopy and ab initio molecular dynamics simulations study shows that proton vacancies in the form of hydroxide/methoxide ions are as relevant for proton transfer between acids and bases as hydrated excess protons (H3O+, H5O2+), thus pointing for a clear demand for refinement of the microscopic picture for aqueous proton transport – in solution as well as in hydrogen fuel cells or transmembrane proteins – away from currently often assumed dominant role of hydrated excess protons. The study is recently published by scientists of the Max Born Institute of Nonlinear Optics and Short Pulse Spectroscopy (MBI) and the Martin-Luther-University Halle-Wittenberg (MLU) in the renowned Journal of the American Chemical Society.
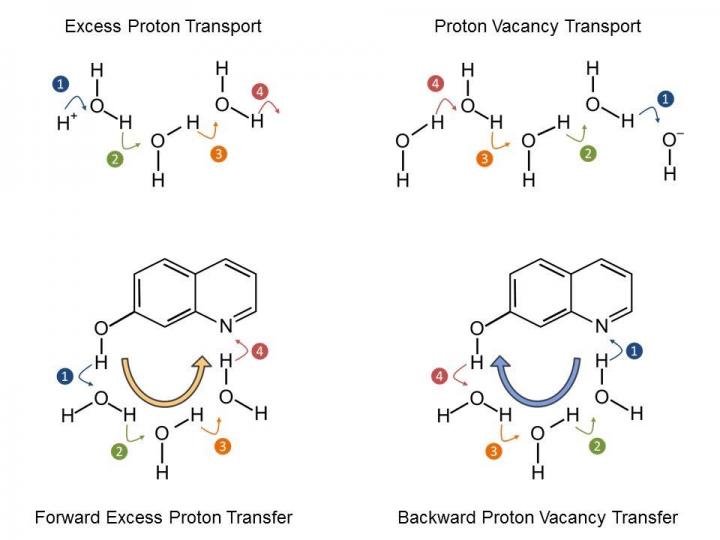
The exchange of protons between two chemical groups (acid-base neutralization) is a textbook chemistry problem for many years. Surprisingly, up to this date new fundamental insight about the elementary steps of proton transport is being obtained. This may well lie in the fact that the elementary steps (by protons or proton vacancies) take place on extremely short time scales, that are not accessible with conventional laboratory techniques (Figure 1). Observation of these elementary reaction steps, as achieved by the research teams of MBI and MLU thus requires direct access to time scales of 1-100 picoseconds (0.000000000001 until 0.0000000001 seconds), necessitating an experimental set-up with an accordingly high time resolution as well as high performance computer systems.
The research teams have jointly studied a particular model system (7-hydroxyquinoline in water/methanol mixtures), where an ultrashort laser pulse triggers the deprotonation of an OH-group and the protonation of a nitrogen atom. The precise chronology of the elementary steps with these class of chemical reactions have remained elusive, leading to numerous speculations. The scientists of the MBI and MLU have now been able to determine that the release of a proton from the OH-group to the solvent is indeed ultrafast, yet the pick-up of a proton by the nitrogen atom is even faster. This results in a transport mechanism of proton vacancies, i.e. of hydroxide/methoxide ions. The elementary reaction steps have been elucidated with time-resolved IR spectra and detailed quantum chemical calculations (see Figure 2).
###
Media Contact
Erik T. J. Nibbering
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




