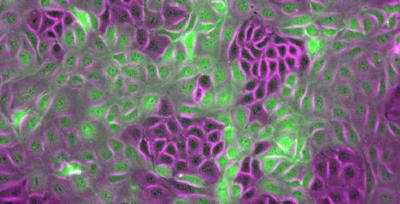
Credit: Amin Doostmohammadi
How do the billions of cells communicate in order to perform tasks? The cells exert force on their environment through movement – and in doing so, they communicate. They work as a group in order to infiltrate their environment, perform wound healing and the like. They sense the stiffness or softness of their surroundings and this helps them connect and organize their collective effort. But when the connection between cells is distrubeddisturbed, a situation just like when cancer is initiated, can appear.
Assistant Professor Amin Doostmohammadi at the Niels Bohr Institute, University of Copenhagen has investigated the mechanics of cell movement and connection in an interdisciplinary project, collaborating with biophysicists in France, Australia, and Singapore, using both computer modelling and biological experiments. The result is now published in Nature Materials.
Amin Doostmohammadi explains: “We need to understand how cells translate this “knowledge from sensing” at the individual cell level and transform it into action on the collective level. This is still kind of a black box in biology – how do cell talk to their neighbors and act as a collective?”
The force of surrounding tissue dictates cell behavior
Individual cells have a contractile mode of motion: they pull on the surface they are located on to move themselves forward. However, cells lining up cavities and surfaces in our body, like the tubes of blood vessels or the cells at the surface of organs, are able to generate extensile forces. They do the opposite, they stretch instead of contract – and they form strong connections with their neighbors. Contractile cells are able to switch to becoming extensile cells, when coming into contact with their neighbors. If, for instance, when contractile cells sense a void or an empty space, like when a wound appears, they can loosen their cell – cell connection, become more individual, and when healing the wound, they form strong connections with their neighbors again, becoming extensile, closing the gap, so to speak.
Weakening cell connection can be the hallmark of cancer initiation
The cells connect to their neighbors by adherens junctions. They connect their internal cytoskeleton to one another and become able to transmit forces through the strong contacts. “So we asked ourselves what would happen if we prohibited the cells from making this strong connection – and it turned out that extensile, strongly connected cells turned into contractile cells with weaker connections. This is significant, because the loss of this contact is the hallmark of cancer initiation. The cells losing contact start behaving more as individuals and become able to infiltrate their surroundings. This process also happens when an embryo develops, but the key difference here is that when the healthy cells have achieved their goal, like forming an organ, they go back to their original form. Cancer cells do not. They are on a one way street”, Amin Doostmohammadi says.
The basic action and reaction of cells are determined by surroundings and communication
How cells “decide” when to go from one form to another is a complicated mix of reacting to their environment, changes in the chemical composition of it, the mechanical stiffness or softness of the tissue – and many proteins in the cells are involved in the process. The key finding of this study is that this reaction to surroundings is constantly shifting: There is a constant cross-talk between cell – surroundings and cell – cell, and this is what determines the actions and reactions of the cells.
Are treatments for cancer within the scope of this new understanding in cell mechanics?
“We must always be careful, when talking about a serious and very complex disease like cancer”, Amin Doostmohammadi says. “But what we can say is that this study brings us one step closer to understanding the basic mechanics of cell behavior, when the cells go from the normal behavior to the aggressive, cancer type cell behavior. So, one of the big questions this study raises is if we might be able to target the mechanics of the cells by some form of therapy or treatment, instead of targeting the DNA or chemical composition of the cells themselves? Could we target the environment instead of the cells? This is basic research, connecting physics and biology, into the mechanics of cell behavior, based on their sensing and responding to the surroundings and coordinating their effort – our improved understanding of this may well lead to new therapies, and there are trials going on at the moment at a preliminary stage”.
###
Media Contact
Amin Doostmohammadi
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




