Credit: College of Engineering, Carnegie Mellon University
Carnegie Mellon University’s He Lab is focusing on noninvasive neuroengineering solutions that not only provide diagnostic techniques, but also innovative treatment options. Their latest research has demonstrated that noninvasive neuromodulation via low-intensity ultrasound can have cell-type selectivity in manipulating neurons.
Parkinson’s Disease, epilepsy and insomnia are just a few of the neurological disorders that use neuromodulation treatment techniques today. Neuromodulation delivers controlled physical energy to the nervous system to treat and improve patients’ quality of life. Current neuromodulation approaches, while effective, bring both drawbacks and limitations.
“Deep brain stimulation, which is highly successful, but an invasive form of electric stimulation through implanted electrodes, is one example of how neuromodulation is being used in a clinical setting today,” explained Bin He, professor of biomedical engineering at Carnegie Mellon University. “Medical professionals have also used noninvasive transcranial magnetic stimulation and transcranial current stimulation, both of which lack the ability to specifically focus on the neuro-circuit level. My group is interested in helping to develop a more effective and completely noninvasive alternative.”
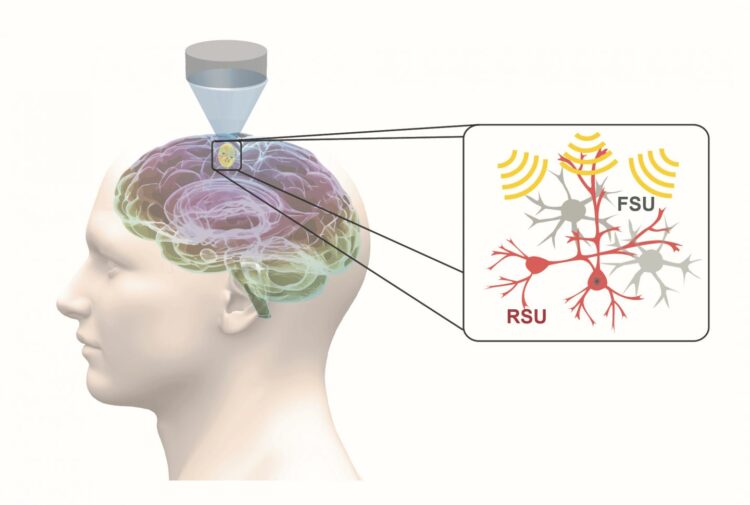
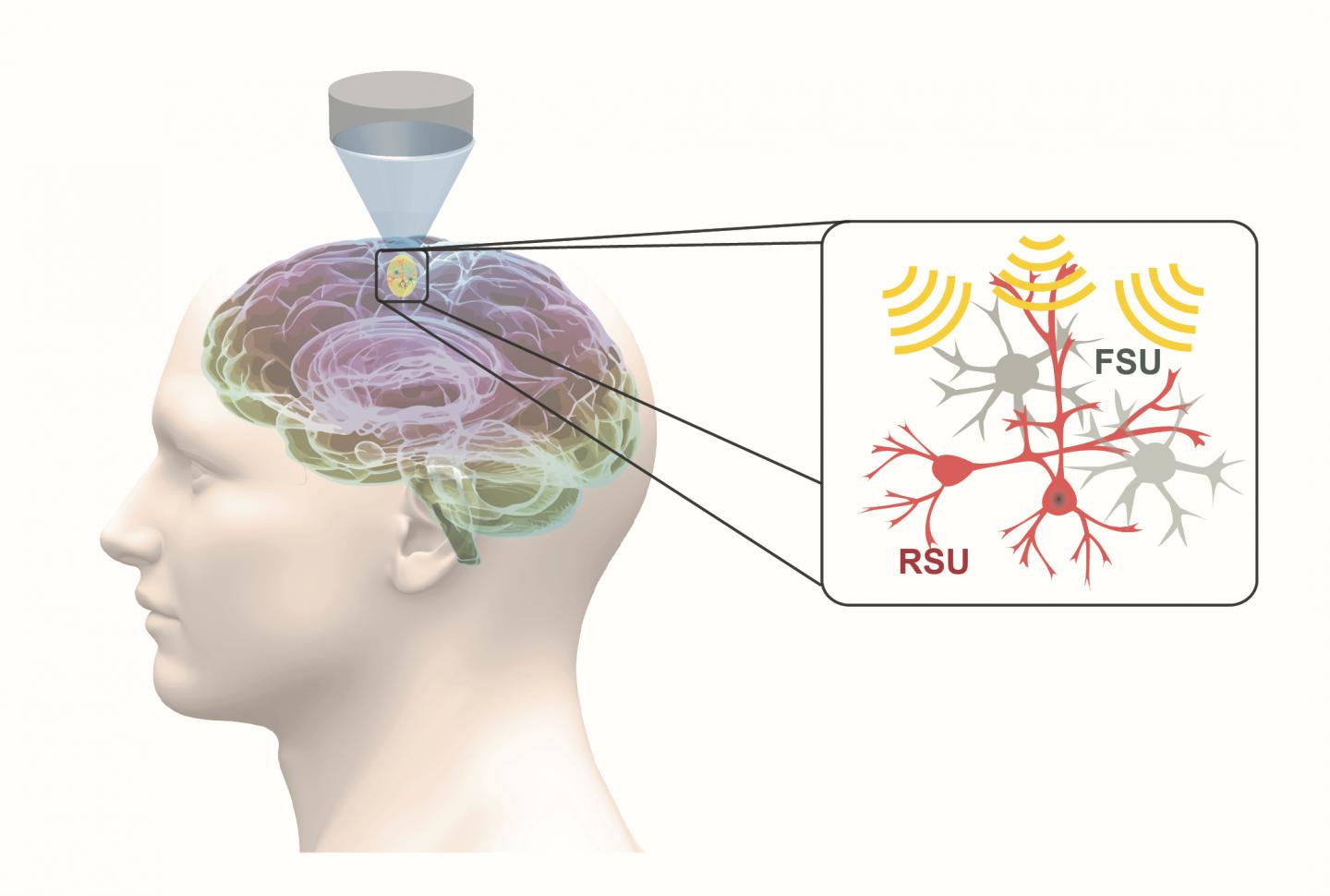
Low-intensity transcranial focused ultrasound, or tFUS, is an emerging and fully reversable neuromodulation technology. It is noninvasive, precise, and it does not require surgery. During tFUS neuromodulation, pulsed mechanical energy is transmitted through the skull, with high spatial resolution and selectivity, at highly-targeted brain regions, which can be steered to elicit activation or inhibition through parameter tuning.
In work recently published in Nature Communications, He’s group demonstrated, for the first time, that specific cell types can be targeted through tFUS neuromodulation. Their study found that excitatory neurons showed high sensitivity to ultrasound pulse repetition frequency, while inhibitory neurons did not.
This finding is significant, because it demonstrates the first capability for a noninvasive neuromodulation technique to modulate a selected cell subpopulation, using a technique that can be directly translated for human use. With the demonstrated capability of tFUS to activate excitatory or inhibitory neurons, future applications may lead to precise targeting of brain circuits using focused ultrasound energy, and activate or inhibit selected sub-populations of neurons by tuning ultrasound parameters.
“As a result of our research, we obtained direct evidence that different neuron populations unequally respond to ultrasound stimulation in the brain,” said Kai Yu, co-first author of the paper and a research scientist in He’s lab at Carnegie Mellon University. “We identified a critical stimulation parameter that is able to tune the balance between excitatory and inhibitory neuronal activities, and we conducted thorough control experiments to support these valuable neuroscience findings.”
The application of this research has broad implications; it’s not just limited to one disease. For many people suffering from pain, depression and addition, He believes non-invasive tFUS neuromodulation could be used to facilitate treatment.
“If we can localize and target areas of the brain using acoustic, ultrasound energy, I believe we can potentially treat a myriad of neurological and psychiatric diseases and conditions,” said He. “This type of treatment option has great potential to shift what doctors study in medical school and go on to practice. Of course, a noninvasive, precise, reversive treatment option also presents endless benefits for patients. My dream would be to make everything noninvasive.”
He’s next goal is to further develop the tFUS neuromodulation technology with increased spatial resolution and focality, and directly test the applicability of tFUS to treat brain conditions in humans.
###
This work was supported in part by the NIH BRAIN Initiative, National Institute of Mental Health, National Institute of Biomedical Imaging and Bioengineering, National Institute of Neurological Disorders and Stroke, and National Center for Complementary and Integrative Health.
Other collaborators on the Nature Communications paper include the co-first author Xiaodan (Rachel) Niu, BME Ph.D. student, and Esther Krook-Magnuson, assistant professor of neuroscience at the University of Minnesota.
Media Contact
Sara Vaccar
[email protected]
Original Source
https:/
Related Journal Article
http://dx.