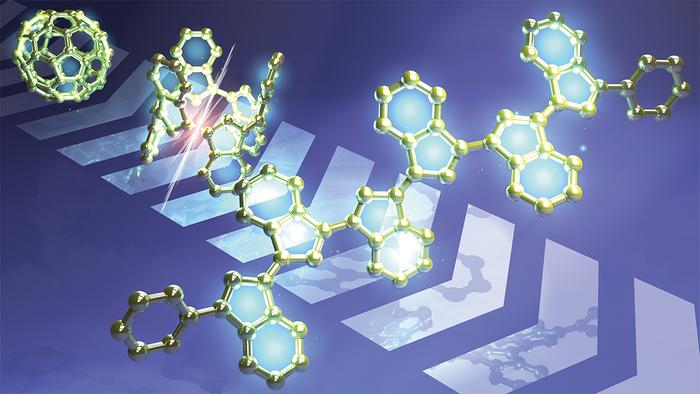
Researchers at Kyoto University in Japan have gained new insights into the unique chemical properties of spherical molecules composed entirely of carbon atoms, called fullerenes. They did it by making flat fragments of the molecules, which surprisingly retained and even enhanced some key chemical properties. The team published their findings in the journal Nature Communications.
Credit: YAP Co., Ltd
Researchers at Kyoto University in Japan have gained new insights into the unique chemical properties of spherical molecules composed entirely of carbon atoms, called fullerenes. They did it by making flat fragments of the molecules, which surprisingly retained and even enhanced some key chemical properties. The team published their findings in the journal Nature Communications.
“Our work could lead to new opportunities in a wide range of applications, such as semiconductors, photoelectric conversion devices, batteries, and catalysts,” says group leader Aiko Fukazawa at the Institute for Integrated Cell-Material Sciences (iCeMS).
Buckminsterfullerene (or simply ‘buckyball’) is a molecule in which 60 carbon atoms are bonded to form a spherical shape. It was named after structural similarities to the geodesic domes designed by the celebrated architect Buckminster Fuller, and its unique structure has continuously attracted the interest of scientists. The buckminsterfullerene and related spherical carbon clusters with different numbers of carbon atoms are colloquially known as fullerenes, after Fuller’s surname. One of their most intriguing characteristics is a capability to accept electrons, a process known as reduction. Because of their electron-accepting character, fullerenes and their derivatives have been extensively investigated as electron-transporting materials in organic thin-film transistors and organic photovoltaics. Nevertheless, fullerenes are an anomalous class of materials compared with any other conventional organic electron-acceptors, due to their robustness toward accepting multiple electrons.
Theoretical chemists have proposed three possible factors that might be behind fullerene’s electron-accepting ability: the high symmetry of the entire molecule, its carbon atoms with pyramidally arranged bonds, and the presence of pentagonal substructures distributed among six-membered rings.
The Kyoto team focused on the influence of the pentagonal rings. They designed and synthesized flattened fragments of fullerene, and experimentally confirmed that these molecules could accept up to an equal number of electrons as the number of five-membered rings in their structure without decomposition.
“This surprising discovery highlights the crucial significance of the pentagonal substructure for generating stable multi-electron accepting systems,” says Fukazawa.
Experiments also revealed that the fragments display enhanced absorbance of ultraviolet, visible, and near-infrared light compared to a more limited absorbance by fullerene itself. This might open new possibilities in photochemistry, such as using light to initiate chemical reactions or developing light sensors or solar-powered systems.
The team will now explore the possibilities their flat fullerene fragments hold in the vast variety of applications associated with electron-transfer processes. It is unusual to get such high electron-accepting ability in molecules composed only of carbon, avoiding the typical requirement to introduce other electron-withdrawing atoms or functional groups onto a carbon-based framework. Going on to explore the effects of incorporating other atoms or chemical groups, however, might yield additional control over and versatility in chemical properties.
“We hope to pioneer the science and technology of what we call super-electron-accepting hydrocarbons, by taking advantage of their high degree of freedom for exploring the effects of structural modifications,” says Fukazawa.
###
DOI: 10.1016/j.ygeno.2022.110372
About Kyoto University’s Institute for Integrated Cell-Material Sciences (iCeMS):
At iCeMS, our mission is to explore the secrets of life by creating compounds to control cells, and further down the road to create life-inspired materials.
https://www.icems.kyoto-u.ac.jp/
For more information, contact:
I. Mindy Takamiya/Christopher Monahan
[email protected]
Journal
Nature Communications
DOI
10.1016/j.ygeno.2022.110372
Article Title
Flattened 1D fragments of fullerene C60 that exhibit robustness toward multi-electron reduction
Article Publication Date
15-May-2023




