BRILLIANCE clinical trial aims to enable sight in people born with a blindness-causing mutation
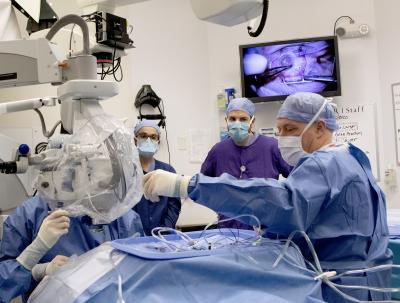
Credit: Oregon Health & Science University
The gene-editing tool CRISPR has been used to address a blindness-causing gene mutation at Oregon Health & Science University for a clinical trial sponsored by Allergan plc and Editas Medicine.
The procedure marks the first time CRISPR has been used to edit human genes within the body, which is also called in vivo gene editing. Previous gene-editing methods have involved editing genetic material after it was removed from the body. The trial’s gene editing approach is designed to be permanent, but not passed onto the offspring of those who receive it. More information is in today’s announcement from the trial sponsors.
Clinicians with OHSU’s Casey Eye Institute performed the procedure for the BRILLIANCE clinical trial, which seeks to repair mutations in the CEP290 gene that cause a rare form of inherited blindness called Leber congenital amaurosis type 10; also known as LCA10 and CEP290-related retinal dystrophy. Most people with the mutation are either born blind or become blind within the first decade of their life.
“Being able to edit genes inside the human body is incredibly profound,” said Mark Pennesi, M.D., Ph.D., who leads OHSU’s involvement in the trial and is the Kenneth C. Swan associate professor of ophthalmology in the OHSU School of Medicine; and, chief of the OHSU Casey Eye Institute’s Paul H. Casey Ophthalmic Genetics Division. “Beyond potentially offering treatment for a previously untreatable form of blindness, in vivo gene editing could also enable treatments for a much wider range of diseases.”
###
Academic institutions currently involved in the trial include: OHSU; Bascom Palmer Eye Institute in Miami, Florida; Massachusetts Eye and Ear in Boston, Massachusetts; and W.K. Kellogg Eye Center at the University of Michigan in Ann Arbor, Michigan.
OHSU’s Casey Eye Institute currently is involved in 14 different clinical trials investigating new genetic treatments for ophthalmic conditions and about 50 vision-related clinical trials overall.
Links:
- BRILLIANCE trial summary at ClinicalTrials.gov: https:/
/ clinicaltrials. gov/ ct2/ show/ NCT03872479 - Allergan/Editas joint news announcement: https:/
/ ir. editasmedicine. com/ news-releases/ news-release-details/ allergan-and-editas-medicine-announce-dosing-first-patient
Related OHSU News stories:
- High hopes for 4-year-old’s vision after gene therapy, https:/
/ (Oct. 16, 2018)news. ohsu. edu/ 2018/ 10/ 16/ releases-20181015-6723986 - OHSU to provide new, FDA-approved gene therapy for a form of blindness, https:/
/ (Dec. 26, 2017)news. ohsu. edu/ 2017/ 12/ 26/ releases-20171226-6662760
Media Contact
Franny White
[email protected]
503-494-4158




