Researchers from Tokyo Medical and Dental University (TMDU) report the construction of a synthetic polymer biomaterial that successfully recapitulates the pancreatic adenocarcinoma microenvironment and could be used to identify novel treatment targets
Credit: Department of Stem Cell Regulation, TMDU
Researchers from Tokyo Medical and Dental University (TMDU) report the construction of a synthetic polymer biomaterial that successfully recapitulates the pancreatic adenocarcinoma microenvironment and could be used to identify novel treatment targets
Tokyo, Japan – One of the biggest challenges in biomedical research is finding a way to capture the complexity of the human body in laboratory-based techniques, to enable them to be investigated accurately. Now, researchers from Japan report an approach for precisely imitating a key feature of aggressive cancers in the laboratory.
In a study published recently in Inflammation and Regeneration, researchers from Tokyo Medical and Dental University (TMDU) have revealed that a synthetic scaffold mimicking the cancer microenvironment could be a powerful tool for understanding cancer progression and treatment.
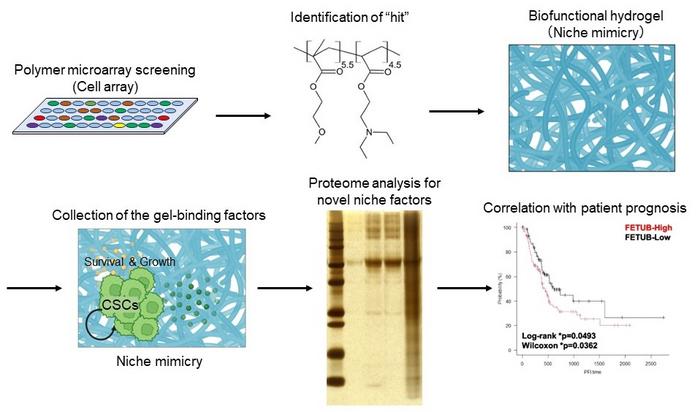
Pancreatic adenocarcinoma is a particularly deadly cancer, in part because it is difficult to detect in the early stages, and in part because of the presence of persistent cancer stem cells (CSCs), which are responsible for metastasis, resistance to treatment, and relapse. These CSCs exist within a specialized cancer microenvironment called a niche, and disrupting this CSC niche could impair cancer progression.
“CSCs communicate with their niche through various cues, such as biological factors, physicochemical factors, and inflammatory factors,” says lead author of the study Yoshitaka Murota. “The complexity of the CSC niche makes it challenging to identify useful treatment targets, particularly in patient samples, so we sought to design a laboratory-based artificial niche that would recapitulate this intricate microenvironment.”
To do this, the researchers applied pancreatic adenocarcinoma cells to an extensive array of synthetic biofunctional materials. They then assessed cellular growth on each of the candidate materials to select the one that most strongly supported growth of CSCs, but not non-CSCs.
“The results were unequivocal,” explains Kouichi Tabu, senior author. “When we embedded the selected polymer in hydrogel to create a niche-mimicking scaffold, it specifically supported substantial growth and proliferation of human pancreatic CSCs.”
Next, the researchers analyzed the serum proteins that were enriched on the hydrogel where the pancreatic cells were growing and compared them to factors known to promote pancreatic cancer. The analysis showed that the niche-mimicking biomaterial trapped multiple serum factors, one of which is closely associated with poor prognosis in patients with pancreatic adenocarcinoma.
“Taken together, our findings show that the polymeric hydrogel that we fabricated accurately mimics the pancreatic CSC niche and can be used to identify therapeutic targets that correlate significantly with patient survival,” says Murota.
Given that CSCs and the cancer microenvironment are virtually universal features of cancer, it is likely that the findings from this study can be applied to other cancer types. Using this type of synthetic polymer material to create artificial CSC niches may help identify important cancer biomarkers and treatment targets in the future.
###
The article, “A niche‑mimicking polymer hydrogel‑based approach to identify molecular targets for tackling human pancreatic cancer stem cells,” was published in Inflammation and Regeneration at DOI: 10.1186/s41232-023-00296-0
Journal
Inflammation and Regeneration
DOI
10.1186/s41232-023-00296-0
Article Title
A niche-mimicking polymer hydrogel-based approach to identify molecular targets for tackling human pancreatic cancer stem cells



