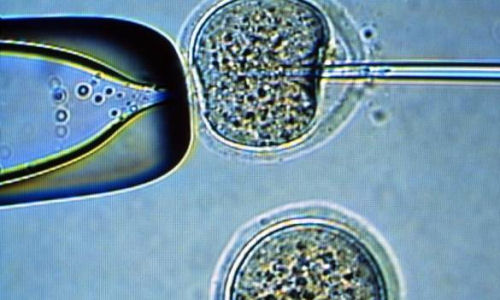
Stem cells made from the skin of adult, infertile men yield primordial germ cells — cells that normally become sperm — when transplanted into the reproductive system of mice, according to researchers at the Stanford University School of Medicine and Montana State University.
The infertile men in the study each had a type of genetic mutation that prevented them from making mature sperm — a condition called azoospermia. The research suggests that the men with azoospermia may have had germ cells at some point in their early lives, but lost them as they matured to adulthood.
Although the researchers were able to create primordial germ cells from the infertile men, their stem cells made far fewer of these sperm progenitors than did stem cells from men without the mutations. The research provides a useful, much-needed model to study the earliest steps of human reproduction.
“We saw better germ-cell differentiation in this transplantation model than we’ve ever seen,” said Renee Reijo Pera, PhD, former director of Stanford’s Center for Human Embryonic Stem Cell Research and Education. “We were amazed by the efficiency. Our dream is to use this model to make a genetic map of human germ-cell differentiation, including some of the very earliest stages.”
A difficult process to study
Unlike many other cellular and physiological processes, human reproduction varies in significant ways from that of common laboratory animals like mice or fruit flies. Furthermore, many key steps, like the development and migration of primordial germ cells to the gonads, happen within days or weeks of conception. These challenges have made the process difficult to study.
Reijo Pera, who is now a professor of cell biology and neurosciences at Montana State University, is the senior author of a paper describing the research, published May 1 in Cell Reports. The experiments in the study were conducted at Stanford, and Stanford postdoctoral scholar Cyril Ramathal, PhD, is the lead author of the paper.
The research used skin samples from five men to create what are known as induced pluripotent stem cells, which closely resemble embryonic stem cells in their ability to become nearly any tissue in the body. Three of the men carried a type of mutation on their Y chromosome known to prevent the production of sperm; the other two were fertile.
The germ cells made from stem cells stopped differentiating in the mice before they produced mature sperm (likely because of the significant differences between the reproductive processes of humans and mice) regardless of the fertility status of the men from whom they were derived. However, the fact that the infertile men’s cells could give rise to germ cells at all was a surprise.
Previous research in mice with a similar type of infertility found that although they had germ cells as newborns, these germ cells were quickly depleted. The Stanford findings suggests that the infertile men may have had at least a few functioning germ cells as newborns or infants. Although more research needs to be done, collecting and freezing some of this tissue from young boys known to have this type of infertility mutation may give them the option to have their own children later in life, the researchers said.
Possible therapies?
“This research provides an exciting and important step for the promise of stem cell therapy in the treatment of azoospermia, the most severe form of male factor infertility,” said Michael Eisenberg, MD, an assistant professor of urology at Stanford and director of the male reproductive medicine and surgery program. “While the study clearly demonstrates the importance that genetics play in spermatogenesis, it also suggests that some of these limitations could potentially be overcome.” Eisenberg often collaborates with Reijo Pera, but he was not an author of the current study.
In 2009, Reijo Pera showed that it is possible to generate functional, sperm-producing germ cells from human embryonic stem cells grown under certain conditions in the laboratory. However, those stem cells, which were made from human embryos that had been donated for research after in vitro fertilization procedures, can be difficult to come by. They also are not useful for couples wishing to have their own genetic children because the child would inherit a portion of the genome of the embryo from which the cells were derived. Furthermore, the process required scientists to engineer the stem cells so they would overexpress several genes, which was necessary to drive the embryonic stem cells to become germ cells.
In contrast, the stem cells made from adult skin required no artificial manipulation. Once implanted into the seminiferous tubules of mice (where the animals’ sperm production takes place), they differentiated into what the scientists termed “germ-cell-like cells,” simply by virtue of the environment in which they were placed. The cells expressed many genes known to be expressed in primordial germ cells, and underwent a genetic reprogramming process called demethylation associated with sperm production.
“We found that the induced pluripotent stem cells were very efficiently driven down that lineage pathway,” said Reijo Pera. “This system has shown that the genetic background of the person from whom the stem cells are derived affects how many primordial germ cells are made. We’re now able to recapitulate that process and begin to study things like gene expression patterns and the distinct steps involved in this process.”
Zeroing in on the problem
Stark differences were seen when stem cells from the fertile and infertile men were compared. The researchers estimated that the cells from the infertile men, who each had a mutation in a region of the genome known as AZF1, were about 50- to 100-fold less efficient than fertile men in their ability to form primordial germ cells.
“Studying why this is the case will help us understand where the problems are for these men and hopefully find ways to overcome them,” said Reijo Pera.
“In addition, it provides very intriguing possibilities for men rendered sterile after cancer treatments,” said Eisenberg. “Being able to efficiently convert skin cells into sperm would allow this group to become biologic fathers. Infertility is one of the most common and devastating complications of cancer treatments, especially for young boys and men.”
The researchers would like to continue their work using non-human primates for transplantation studies. They’re also interested in exploring the effect of other types of infertility-associated mutations on germ cell production.