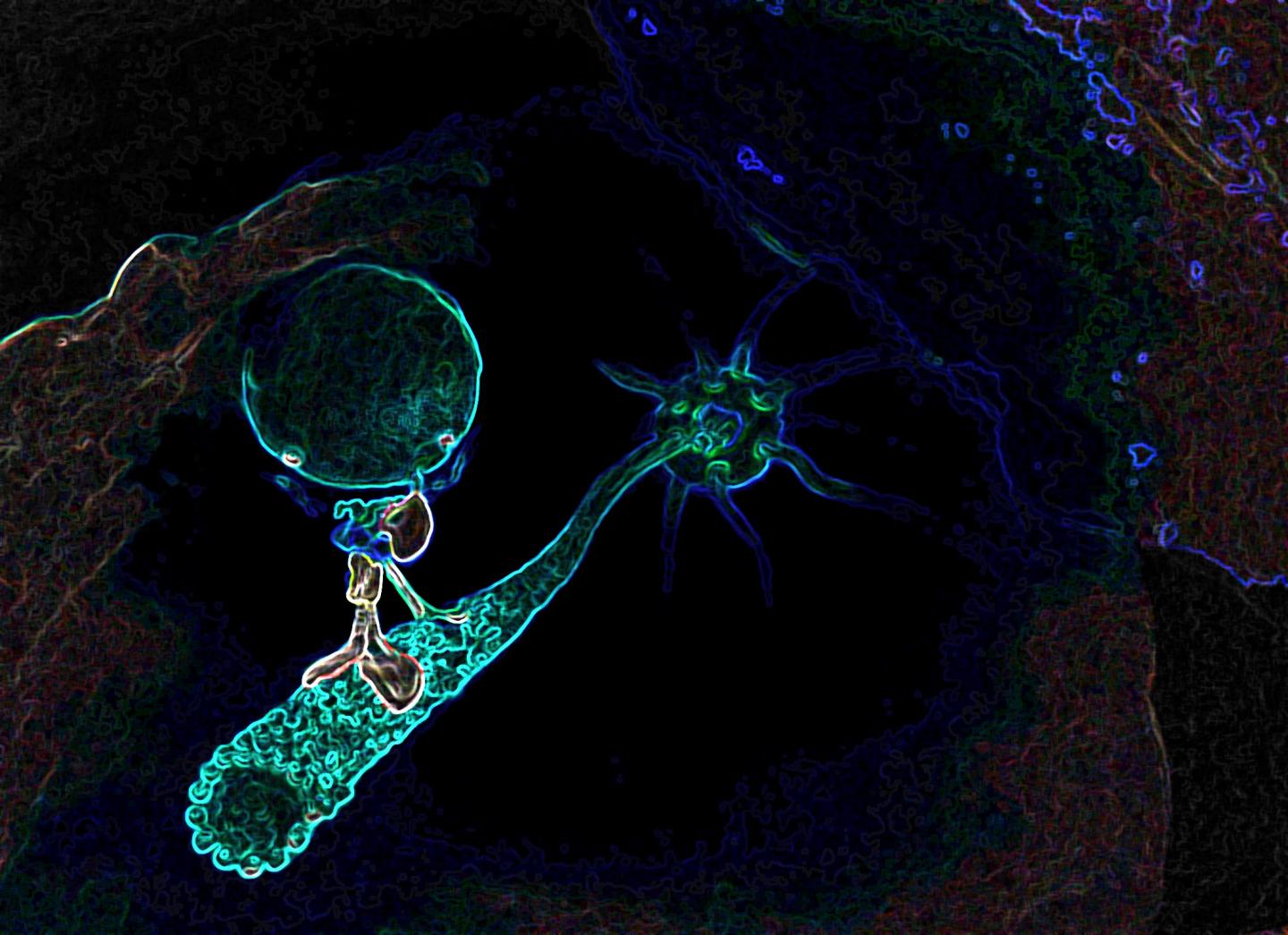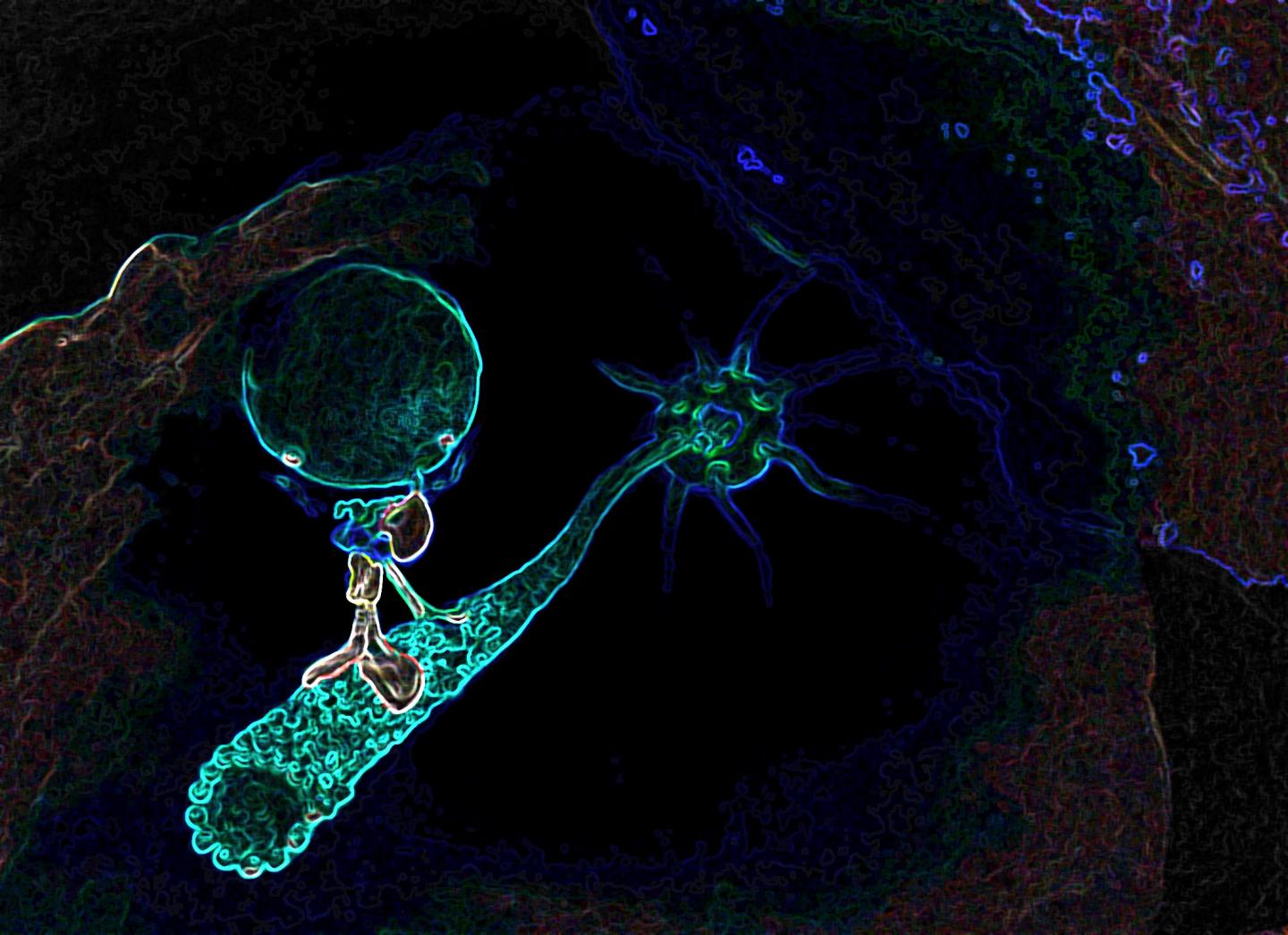
Credit: K. Boztug and E. Salzer; CeMM/Wolfgang Däuble
A multi-institutional, international team of scientists has discovered the genetic cause and biological mechanisms linked to a new human immunodeficiency. The study, which is published in Nature Immunology, also identifies a potential treatment.
Dr. Kaan Boztug, who is the senior author of the paper and an investigator at the CeMM Research Institute for Molecular Medicine of the Austrian Academy of Sciences, the Ludwig Boltzmann Institute for Rare and Undiagnosed Diseases and the Medical University at Vienna, led the team of dozens of scientists from various institutions across the world, including Baylor College of Medicine.
First author Dr. Elisabeth Salzer, postdoctoral fellow in the Boztug lab, identified a 12-year-old patient who suffered repeated life-threatening infections since his birth. Three of the patient's six siblings had died within their first two years seemingly of a similar disorder. The researchers suspected that a genetic condition might be linked to the patient's inability to fight infection.
"Our analyses of the patient's and his parents' genomes indeed confirmed that the boy's disorder had a genetic cause," said Salzer.
The genetic cause is an error in the gene RASGRP1 that renders the gene inactive. This type of mutation had never been reported before. The healthy parents and the healthy siblings carry one mutated copy of the gene and one normal copy that compensates for the faulty gene. The patient, on the other hand, inherited one mutated copy from each parent.
The patient presented with a primary immunodeficiency that involves a new combination of immune defects in essential members of the immune system, T cells, B cells and Natural Killer cells. Until now, the role RASGRP1 plays in the immune system had not been studied in humans.
To determine the mechanisms that might lead to the patient's inability to fight infections, the Vienna group collaborated with the lab of Dr. Jordan Orange at Baylor. Orange is professor and section head for immunology, allergy and rheumatology in pediatrics at Baylor and the director of the Center for Human Immunobiology at Texas Children's Hospital.
"The clinical characteristics of the patient suggested that some of the defective immune mechanisms of his condition were of the type we study in our lab," said Orange. "We applied our expertise on quantitative and high-resolution imaging to study the effects of the RASGRP1 mutation in Natural Killer cells."
The Baylor group found that the gene RASGRP1 plays a role in dynein functions in Natural Killer cells. Dynein is a motor protein; it moves things around inside cells.
"Like motorized vehicles carrying people around a city, motor proteins such as dynein transport items inside cells where they need to go," said Orange. "Natural Killer cells rely heavily on the dynein transportation system to secrete poisons onto diseased cells – cells infected with viruses, for instance – to destroy them. In this disease, the 'motorized vehicles' are not working properly; the poison cannot be transported to the virus-infected cells and the patient cannot get rid of infections."
The studies from the Orange lab provided a functional link between the defects in Natural Killer cells and dynein, which in combination with other observations led the Austrian team to try the drug lenalidomide to treat the patient. The drug showed potential to reverse some of the effects of RASGRP1's mutation.
"The whole process from the discovery of a gene defect as the cause of a rare disease to the exploration of the disease-causing mechanism to the development of a personalized therapy does much more than helping the affected patients," said Boztug. "Virtually every case – such as the immunodeficiency of this young patient – provides profound new insights into the human organism and paves the way toward a future precision medicine."
"This work was the result of a tremendous collaboration among international groups of researchers and with the family of the patient," said Orange. "Our work at Baylor and Texas Children's Hospital continues to include us among the world leaders in the study of primary immnodeficiencies and their biological underpinnings."
###
Other contributors to this work included Deniz Cagdas, Miroslav Hons, Emily Margaret Mace, Wojciech Garncarz, Özlem Yüce Petronczki, René Platzer, Laurène Pfajfer, Ivan Bilic, Sol A Ban, Katharina L. Willmann, Malini Mukherjee, Verena Supper, Hsiang Ting Hsu, Pinaki P. Banerjee, Papiya Sinha Somasundaram, Fabienne McClanahan, Gerhard J. Zlabinger, Winfried Pickl, John G. Gribben, Hannes Stockinger, Keiryn L. Bennett, Johannes B. Huppa, Loïc Dupré, Özden Sanal, Ulrich Jäger, Michael Sixt and Ilhan Tezcan. Mace is an Assistant Professor of Pediatrics at Baylor, Mukherjee an Instructor and Hsu and Somasundaram are doctoral students in Baylor's Nationally Ranked Immunology Graduate Program.
This work was supported by the European Research Council under the European Union's Seventh Framework Programme (FP7/2007-2013) / ERC grant agreement n° 310857, the Vienna Science and Technology Fund through project LS14-031, an unrestricted research grant from Celgene Austria, the National Institutes of Health (R01AI067946), and fellowships from the Boehringer Ingelheim Fonds, the Austrian Science Fund Project M1809-B19, and the French Agence Nationale de la Recherche (ANR-13-BSV1-0031).
Media Contact
Jeannette Jimenez
[email protected]
713-798-4710
@bcmhouston
https://www.bcm.edu/news
The post Faulty RASGRP1 gene causes newly discovered human immunodeficiency appeared first on Scienmag.