Credit: ©Science China Press
In recent years, rechargeable magnesium batteries (RMBs) have attracted a growing number of researchers in the field of electrochemical energy storage systems due to several inherent strengths. First of all, Mg metal possesses higher abundance in earth crust and volumetric capacity (3833 mAh cm-3) compared with metallic lithium. More importantly, air/moisture stability and dendrite-free morphology upon cycling can provide considerable merits over the reviving lithium metal batteries (LMBs) for large-scale energy storage systems and electric vehicles. However, the uppermost problem stems from the fact that Mg metal anodes with intrinsically high activity are apt to react with conventional electrolyte components (solvents or salts), forming a passivation film on the surface (especially at high current densities), which impedes the conduction of Mg ions in the interphase. Although significant progress has been made in designing various electrolyte systems, rare research concerning Mg anode modification was proposed.
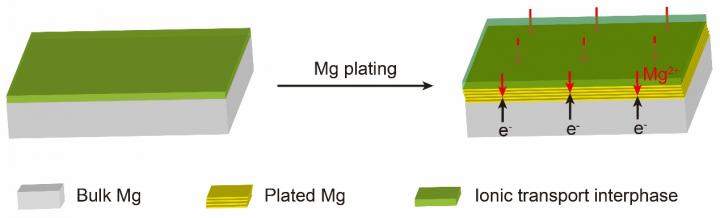
In this article, researchers report a modified Mg metal anode via a safe, facile and effective method to address the issue of irreversible plating/stripping behavior in a conventional electrolyte (Mg(TFSI)2/DME electrolyte). The modified Mg anode was prepared by a facile surface ion-exchange reaction in SnCl2-DME solution, forming a coating layer comprised of tin-based compounds (e.g. Mg2Sn, Sn, etc.) and electronically insulating halides.
Upon cycling, the coating layer could remain compositionally and structurally invariant. Besides, the interfacial resistance and ion transport activation energy were sharply reduced and fast ion diffusion kinetics was performed on the modified Mg anodes. For the artificial layers, the Sn-based compounds provide fast ion transport conduit for Mg ions, showing a high diffusion coefficient of Mg2+, and the insulating halides offer potential gradient to drive Mg ions to electrodeposit under the coating film and prevent plating on the surface. The synergistic effects of Sn-based compounds and insulating halides lead to better electrochemical performance in RMBs. The symmetric Mg/Mg cells with modified Mg anodes exhibit a quite low overpotential (0.2 V) and an ultralong lifespan over 4000 cycles (1400 h) even at a high current density of 6 mA cm-2, which is the most excellent performance to the best of our knowledge. Moreover, cells with modified electrodes paired with TiS2 cathode can be cycled at a current density of 10 mA g-1.
Indeed, the stable artificial layer enable fast ionic transport and reversible plating/stripping process at high current densities in a conventional electrolyte. Researchers perceive that this work can stimulate more research on modifying Mg anodes with other available Mg2+-conducting metals or alloys through ex situ or in situ methods. It should have significant applications in rechargeable magnesium batteries.
###
This research received funding from National Natural Science Foundation of China, Natural Science Foundation of Tianjin, China.
See the article:
Ruijing Lv, Xuze Guan, Jiahua Zhang, Yongyao Xia and Jiayan Luo
Enabling Mg Metal Anodes Rechargeable in Conventional Electrolytes by Fast Ionic Transport Interphase
Natl Sci Rev (October 2019) nwz157
https:/
Media Contact
Jiayan Luo
[email protected]
Related Journal Article
http://dx.




