Shared decision-making seen as an important instrument to obtain better research results and gain support and acceptance from the wider public, according to a new position paper published in the Journal of Neuromuscular Diseases
Credit: CHEO Research Institute in Ontario (Canada)
Amsterdam, February 6, 2019 – The old-fashioned paternalistic relationship between doctors and patients has gradually evolved into a more collaborative one in the era of patient-centered medicine. Shared decision-making (SDM), in which doctors and patients jointly decide on treatment or care, has emerged as a gold standard model of healthcare. Yet considerably less attention has been given to obtaining the patient’s perspective on neuromuscular research on such matters as research objectives, study design, or even consent. A position paper in the Journal of Neuromuscular Diseases describes conclusions reached at an international workshop that focused on finding creative solutions to integrate and enhance the patient’s point of view in neuromuscular research.
“I need to revise my grant application to include patient perspective in my research project!” commented attendee Valeria Sansone, MD, Director of the NEMO Centre, Milan (Italy). Dr. Sansone was one of 45 clinicians, healthcare professionals, researchers, patients, caregivers, and representatives from regulatory agencies and pharmaceutical companies from 15 countries who attended this international workshop. The gathering was convened by the European Neuromuscular Centre (ENMC) upon its 25th anniversary and was held in Milan, Italy from January 19-20, 2018.
“The ENMC, a group of patient organizations for neuromuscular disorders, convened this workshop to familiarize itself with the concept of SDM, which is new for most patients and researchers in the neuromuscular field,” noted Ellen Sterrenburg, PhD, Chair of the ENMC Executive Committee.
The Workshop recommendations include higher patient involvement in research activities related to neuromuscular diseases: clinical trials, sample biobanks/patient registries, and regulatory processes. Educational, structural, and cultural changes need to be implemented by all stakeholders: patients and patient organizations, healthcare professionals, and regulatory bodies.
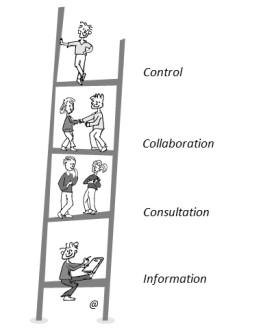
In an introductory talk, Ingeborg Meijer, PhD, of the Center for Science and Technology Studies (CWTS) of the University of Leiden and Spierziekten Nederland, Baarn (The Netherlands) set the tone of the Workshop by presenting a graphic conceptualizing the levels of patient involvement in decision processes related to health called the “ladder of participation.”
“There is a need for a change in culture in many medical research disciplines in order to succeed in the empowerment of patients, their families, and advocates, but this change should be adopted also by researchers, doctors, and all other professionals,” explained Dr. Meijer.
During the session on biobanks and registries, Hanns Lochm?ller of the CHEO Research Institute in Ontario (Canada) said, “Rare disease research centers offer an ideal setup to implement and integrate patient involvement at all levels.” He pointed to several examples of patient co-creation in research programs such as the UK myotonic dystrophy registry, which is patient driven but professionally supported. In this registry, the patient initiates the registration and names a doctor to enter the patient’s clinical data, eliminating possible biases of past registries. Patients have also played a significant role in the TREAT-NMD global registries and developing the International Charter of principles for sharing bio-specimens and data. “Patient commitment is facilitated when registries and researchers commit to reporting research results back to the data providers, the patients, and families. A challenge is educating scientists to be flexible and change their research protocols according to the priorities of the research participants,” explained Dr. Lochm?ller, co-Editor-in-Chief of the Journal of Neuromuscular Diseases.
Patient participation in biobanks would also benefit from SDM. To accomplish this, the ENMC Workshop provided specific educational (e.g., “the consent form should be updated to accommodate modern techniques applied to cell lines and biopsies and written together with patient organizations to ensure understanding”), cultural (e.g., “patients who donate data or samples are to be seen as partners in research and should be aware of this role”), and structural recommendations (e.g., “research information should go back to patients in the form of regular newsletters and updates”).
In a session on clinical trials, participants agreed that involvement of persons with NMD (or patient organizations) in clinical trials, as experts of their own disease, should happen in a proactive way from the beginning and through discussion of trial design options. As an illustration, Baziel van Engelen, MD, PhD, Professor of Neuromuscular Diseases at Radboud University Nijmegen Medical Centre and former ENMC Research Director, presented his experience with patient participation in the OPTIMISTIC clinical trial for myotonic dystrophy type 1. A unique feature of the trial design was asking each patient which determinant of health status the individual wanted to change. “The concept of promoting health (vs. decreasing the disease) and treating the patient (vs. treating the disease) led in this case to an impressive retention level throughout the study and to very high satisfaction of the patients involved,” said Prof. Dr. van Engelen.
Patient input may come from individual patients or patient organizations, such as EURORDIS and those that are condition-specific. Several initiatives are in place to help patients achieve relevant competencies and encourage those who want to take on the responsibilities of being knowledgeable patient representatives. “Having the patient voice in a clinical trial is not only feasible, it should be a logical and natural thing,” commented Dr. Lochm?ller.
###
Media Contact
Diana Murray
[email protected]
718-640-5678
Related Journal Article
http://dx.




