Credit: © DIfE
Mice with a strong tendency to obesity already exhibit epigenetic changes at six weeks of age, inducing the liver to amplify its production of the enzyme DPP4 and release it into the circulation. Over the long term, this favors the development of a fatty liver. Such changes in DNA methylation are also detectable in humans with fatty liver and suggest a similar causal chain. These are the results of a study of an international research team led by Annette Schürmann, Robert Schwenk, Christian Baumeier and Sophie Saussenthaler of the German Institute of Human Nutrition (DIfE), a partner of the German Center for Diabetes Research (DZD).
The team, which includes diabetes researchers from Finland, Sweden and France, has now published its results in the journal Diabetes (Baumeier et al. 2017; https://doi.org/10.2337/db15-1716).
DPP4* is an enzyme that inhibits the action of important intestinal hormones of the glucose metabolism. Various studies have shown that high blood glucose levels stimulate the body's production of the enzyme. In particular, people with non-alcoholic fatty liver disease** have high DPP4 levels in the liver and in the blood. Until now, however, it was unclear whether increased DPP4 is a consequence or a trigger of fatty liver disease.
To find answers to these questions, the scientists studied the gene regulation of the DPP4 gene in mice that are prone to obesity. Similar to identical twins, all animals of this breeding line are genetically identical. Nevertheless, some of the mice gain much more weight under the same high-fat diet and at the adult age of about 20 weeks develop a fatty liver. This suggests that the differences in weight development are due to epigenetic effects.
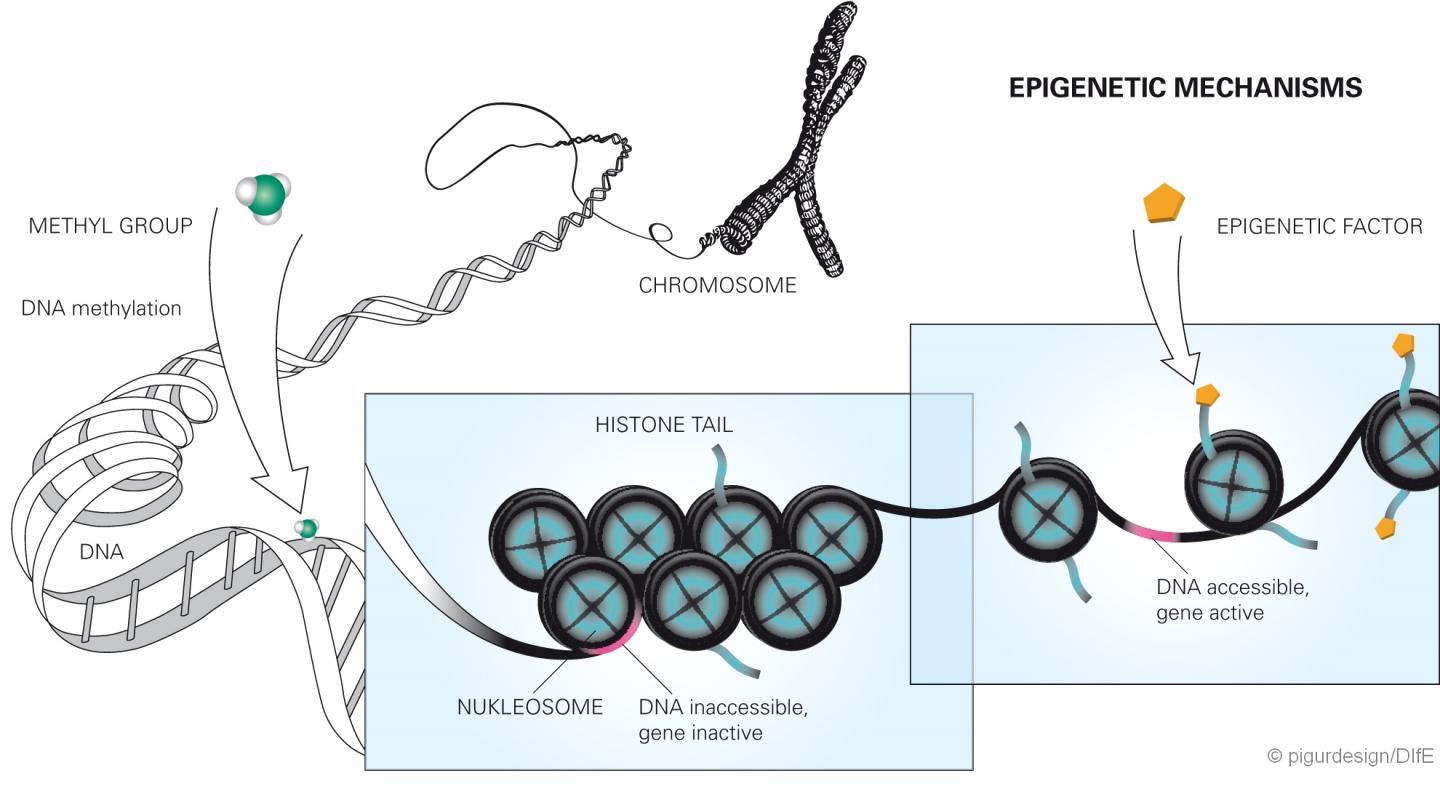
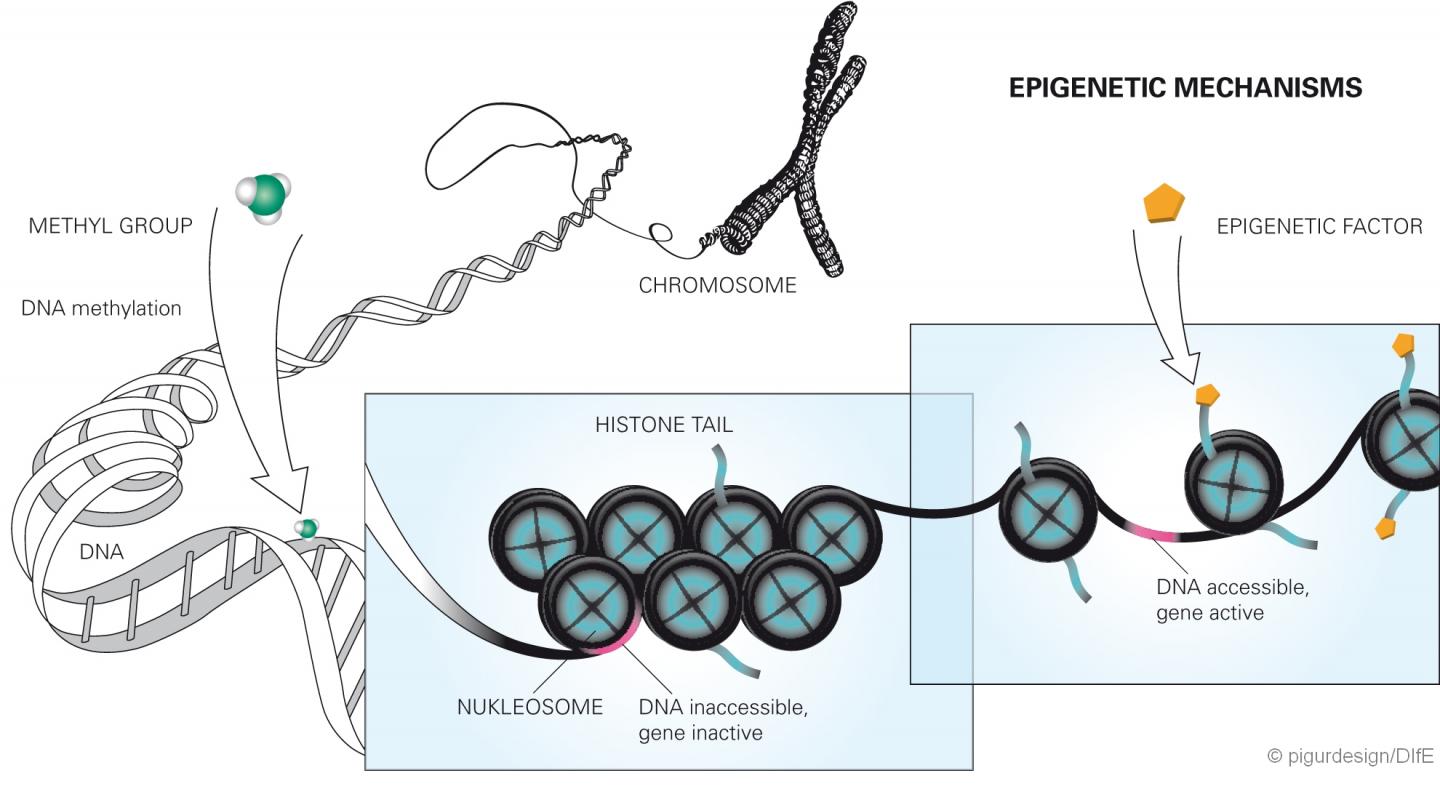
In their study, the researchers showed that already at the age of six weeks in the mice with a rapid weight gain, the DPP4 gene was less methylated at four specific loci, i.e. epigenetically altered, compared to the other mice. As a result, the enzyme synthesis in the liver as well as the enzyme concentrations in the blood increased significantly, depending on the blood glucose level, even before the animals developed a fatty liver. "Perhaps the methylation of the gene can be compared with a dimming switch, which regulates the transcription of the gene and thus the amount of the enzyme formed. If the sites in the gene are methylated, the DPP4 synthesis in the liver cells is 'dimmed', that means reduced and reversed," said Christian Baumeier, the first author of the publication. Furthermore, the scientists observed that later only those adult animals had a fatty liver that exhibited higher DPP4 level in the liver due to reduced methylation. "Our results clearly show that the increased concentrations of DPP4 in the liver and blood that were measured in the obese animals were not the consequence of a fatty liver. Rather the opposite was true, the altered epigenetic regulation of the gene was responsible for the development of the fatty liver," added Sophie Saussenthaler, who shared the first authorship with Baumeier.
As further analyses of the scientists have shown, the DPP4 gene in human liver is regulated by epigenetic changes just as in mice. In tissue samples from patients with severe fatty liver disease, the gene was less methylated. The degree of fat content in the liver correlated with the degree of DPP4 gene methylation and the amount of enzymes produced by the liver.
"Taken together, our results indicate that the epigenetic changes of the DPP4 gene associated with obesity have a negative effect on the liver metabolism already in young people, long before a fatty liver develops," said Annette Schürmann, leader of the study and head of the Department of Experimental Diabetology at DIfE. She continued: "Therefore, in further studies, we should investigate how and at what point DPP4 inhibitors*** can be used in diabetes therapy to prevent the development of non-alcoholic fatty liver disease."
###
Source: Christian Baumeier, Sophie Saussenthaler, Anne Kammel, Markus Jähnert, Luisa Schlüter, Deike Hesse, Mickaël Canouil, Stephane Lobbens, Robert Caiazzo, Violeta Raverdy, François Pattou, Emma Nilsson, Jussi Pihlajamäki, Charlotte Ling, Philippe Froguel, Annette Schürmann and Robert W. Schwenk: Hepatic DPP4 DNA Methylation Associates With Fatty Liver; Diabetes 2017 Jan; 66(1): 25-35.
Background Information
Epigenetics is a relatively young research area. It investigates altered gene functions that cannot be attributed to a change in the DNA sequence, but can still be inherited. Recent studies have increasingly suggested that diet as an environmental factor can also have a lasting effect on the activity of genes, e.g. through chemical changes of the DNA nucleotides. These include methylations. These occur when methyl groups bind to the DNA. This can either impede or facilitate the activation of the genes. The direct methylation of the DNA then permanently changes the gene expression when it occurs in control regions of genes (so-called CpG islets) made accessible by the modification of the histones. In the current study, the researchers found that both the human and mouse DPP4 gene is less methylated when the subjects were heavily overweight and developed a fatty liver. Since the methylation in this case makes the transcription of the gene more difficult, the demethylation (decrease of the degree of methylation) leads to overexpression of the gene in overweight/obese individuals. Both methylation and demethylation can be regarded as epigenetic change.
* DPP4 is the acronym for dipeptidyl peptidase 4. The enzyme cleaves among others the intestinal hormones (incretins) Glucagon-like peptide-1 (GLP-1) and Gastric inhibitory polypeptide (GIP), which as a result lose their effect. This leads to high blood glucose levels, and the function of the insulin-producing cells of the pancreas is adversely affected.
** Non-alcoholic fatty liver disease (NAFLD) is now the most common chronic liver disease in Europe and in the U.S. and a frequent concomitant disease of overweight/obesity and type 2 diabetes. Without treatment, it can develop from fatty liver into liver cirrhosis, which can have life-threatening consequences. It is possible to reverse the effects of NAFLD, whereby weight loss plays the most important role in the treatment (Source: Deutsches Ärzteblatt; Vol. 111; Issue 26; June 27, 2014).
*** DPP4 inhibitors are already used as a drug in diabetes therapy to prolong the action of the two endogenous incretins GLP-1 and GIP. Their aim is to increase insulin secretion after food intake in people with type 2 diabetes.
The German Institute of Human Nutrition Potsdam-Rehbrücke (DIfE) is a member of the Leibniz Association. It investigates the causes of diet-related diseases in order to develop new strategies for prevention and therapy and to provide dietary recommendations. Its research focus includes the causes and consequences of the metabolic syndrome, which is a combination of obesity, high blood pressure, insulin resistance and lipid metabolism disorder, as well as the role of diet in healthy aging and the biological basis of food choices and eating habits. More information at http://www.dife.de. In addition, the DIfE is a partner of the German Center for Diabetes Research (DZD), which was founded in 2009 and has since been funded by the BMBF. More information on the DZD can be found at http://www.dzd-ev.de.
The Leibniz Association connects 91 independent research institutions that range in focus from the natural, engineering and environmental sciences via economics, spatial and social sciences to the humanities. Leibniz Institutes address issues of social, economic and ecological relevance. They conduct knowledge-driven and applied basic research, maintain scientific infrastructure and provide research-based services. The Leibniz Association identifies focus areas for knowledge transfer to policy-makers, academia, business and the public. Leibniz Institutes collaborate intensively with universities – in the form of "WissenschaftsCampi" (thematic partnerships between university and non-university research institutes), for example – as well as with industry and other partners at home and abroad. They are subject to an independent evaluation procedure that is unparalleled in its transparency. Due to the institutes' importance for the country as a whole, they are funded jointly by the Federation and the Länder, employing some 18,600 individuals, including 9,500 researchers. The entire budget of all the institutes is approximately 1.7 billion EUR. More information is available at http://www.leibniz-gemeinschaft.de.
Contact:
Prof. Dr. Annette Schürmann
Department of Experimental Diabetology
German Institute of Human Nutrition
Potsdam-Rehbruecke (DIfE)
Arthur-Scheunert-Allee 114-116
14558 Nuthetal/Germany
phone: +49 (0)33200 88-2368
email: [email protected]
Dr. Christian Baumeier
Department of Experimental Diabetology
German Institute of Human Nutrition
Potsdam-Rehbruecke (DIfE)
Arthur-Scheunert-Allee 114-116
14558 Nuthetal/Germany
phone: +49 (0)33200 88-2505
email: [email protected]
Sophie Saussenthaler
Department of Experimental Diabetology
German Institute of Human Nutrition
Potsdam-Rehbruecke (DIfE)
Arthur-Scheunert-Allee 114-116
14558 Nuthetal/Germany
phone: +49 (0)33200 88-2572
email: [email protected]
Media Contact:
Dr. Gisela Olias
Head, Press and Public Relations
German Institute of Human Nutrition
Potsdam-Rehbruecke (DIfE)
phone: +49 33200 88-2278/-2335
email: [email protected]
or [email protected]
http://www.dife.de
Media Contact
Birgit Niesing
[email protected]
49-893-187-3971
@diabresearch
http://www.dzd-ev.de
############
Story Source: Materials provided by Scienmag