Noble metals have long been considered invaluable for their roles in catalyzing chemical reactions. Among these, platinum stands out due to its exceptional ability to facilitate hydrogenation processes, where hydrogen atoms are added to molecules, creating significant advancements in industrial chemistry. Researchers at the University of California, Davis, have recently made strides in enhancing the stability and efficiency of platinum catalysts through innovative methods involving nanoscale confinement. This development could revolutionize the way catalytic processes are performed in various industrial applications.
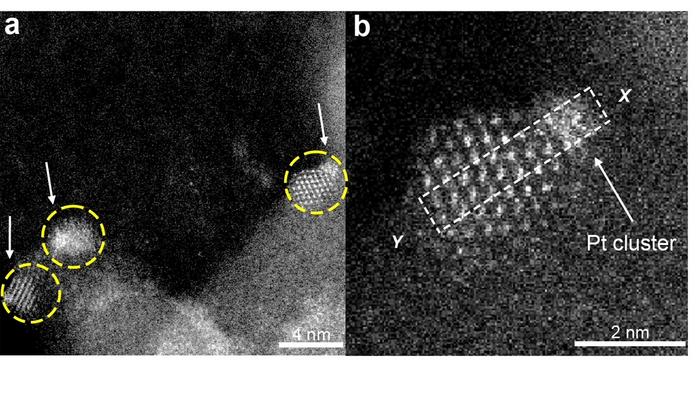
Platinum’s effectiveness as a catalyst is largely attributed to its unique atomic structure, particularly when organized into clusters containing a few atoms. Evidence from recent studies suggests that these small clusters outperform both single platinum atoms and larger nanoparticles in catalyzing hydrogenation reactions. One of the significant challenges is that when these platinum clusters are formed, they have a tendency to coalesce into larger formations. This clumping reduces their overall catalytic efficiency, which can hinder chemical reactions that depend on the presence of these tiny clusters.
The research team, under the guidance of the esteemed Professor Bruce Gates, zeroed in on a novel approach to mitigate this inefficiency. They turned to the idea of ‘trapping’ platinum clusters within tiny islands made from cerium oxide, which are themselves supported on a silica surface. Each island acts like a micro-reactor, providing a controlled environment where the platinum clusters can remain stable during catalytic processes. This cutting-edge strategy not only prevents the clusters from aggregating but also enhances their catalytic activity.
Yizhen Chen, a key contributor to this research and a former postdoctoral scholar within the Gates Catalysis Research Group, further elaborated on the effectiveness of their method. The team’s efforts culminated in the successful production of these platinum clusters, which exhibited notable catalytic activity in hydrogenation reactions involving ethylene. Interestingly, the clusters were found to maintain their stability even under rigorous conditions typically encountered during industrial reactions.
This pioneering work opens new avenues for the production of platinum catalysts that are not only highly efficient but also robust. With the ability to confine these clusters effectively, researchers are optimistic about the broader implications for the chemical industry, especially in processes where stability and efficiency are imperative. The findings of this groundbreaking study were disseminated in a comprehensive report published on January 17 in the reputable journal, Nature Chemical Engineering.
Support for the research came from various esteemed institutions, including grants from the U.S. Department of Energy, the U.S. National Science Foundation, and the National Natural Science Foundation of China. This financial backing underscores the importance and potential impact of this research in advancing catalysis technology and industrial chemistry applications.
A significant aspect of the research process involved rigorous experimental studies designed to explore the effectiveness of the confinement strategy on the platinum clusters. This experimental approach enabled the team to draw comparisons between the performance of confined clusters and traditional catalyst systems, thus showcasing the advantages of their innovative method. The experimental results highlighted how the confined environment plays a pivotal role in enhancing both the reaction rates and product yields.
Moreover, the implications of this research extend beyond just the realm of hydrogenation. As chemical reactions become increasingly central to various industrial processes—from pharmaceuticals to materials science—the development of stable catalyst systems could vastly improve efficiency and safety. This electrochemical route holds promise not only for optimizing current methodologies but also for paving the way towards new catalytic processes that are yet to be explored.
The research findings indicate a clear pathway for further investigation into the stability and activity of various metal clusters when confined in such a manner. Future studies could involve exploring other metals alongside platinum, potentially leading to a new generation of catalyst systems tailored for specific chemical reactions and processes. By combining materials science with advanced catalytic chemistry, the research team aims to unlock additional functionalities that may lead to unprecedented efficiencies in industrial applications.
Additionally, as the demand for cleaner and more sustainable chemistries rises globally, these advancements in catalyst technologies come at a crucial moment. The need for efficient catalysis cannot be overstated; it directly influences energy consumption, waste production, and the overall sustainability of chemical production processes. Thus, the work conducted at UC Davis not only enhances understanding at the atomic level but also provides practical solutions to real-world challenges in the chemical landscape.
In summary, the innovative approach of confining platinum clusters in nano-sized ceramic islands represents a significant leap forward in catalyst development. The implications of this research promise to redefine traditional practices in industrial chemistry, highlighting the critical balance between efficiency and stability. As these findings continue to gain traction in the scientific community, they could inspire further innovative strategies that leverage the unique properties of noble metals in practical applications.
Ultimately, this research demonstrates the power of combining theoretical science with practical applications to create tangible benefits in industrial processes. It exemplifies collaboration across institutions and the collective effort to propel the field of catalysis into a more efficient and sustainable future.
Subject of Research:
Article Title:
News Publication Date:
Web References:
References:
Image Credits:
Keywords
Noble metals, Platinum, Catalyst efficiency, Hydrogenation, Nanostructures, Cerium oxide, Catalysis, Chemical engineering.
Tags: advancements in catalytic processesatomic structure of platinumchallenges in platinum cluster formationefficiency of catalytic reactionshydrogenation processes with platinuminnovative methods in industrial chemistrynanoislands for catalyst stabilitynanoscale confinement in catalysisplatinum catalysts enhancementProfessor Bruce Gates researchresearch on noble metal catalystsstability of platinum clusters