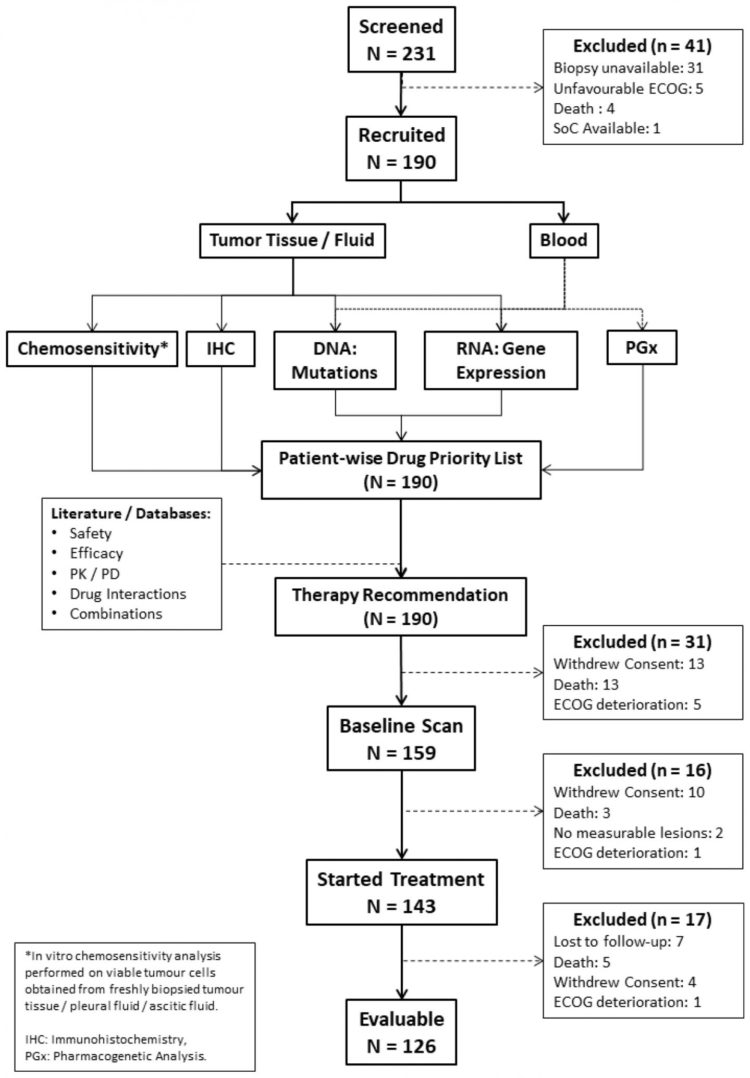
RESILIENT was a single arm, open label, phase II/III study to test if label agnostic therapy regimens guided by Encyclopedic Tumor Analysis can offer meaningful clinical benefit for patients with relapsed refractory metastatic malignancies
Credit: Ajay Srinivasan, email: [email protected]
RESILIENT was a single arm, open label, phase II/III study to test if label agnostic therapy regimens guided by Encyclopedic Tumor Analysis can offer meaningful clinical benefit for patients with relapsed refractory metastatic malignancies.
Patients with advanced refractory solid organ malignancies where disease had progressed following 2 lines of systemic treatments were enrolled in the trial.
At study completion, Disease Control was observed in 114 out of 126 patients evaluable per protocol and Disease Progression in 12 patients.
Dr. Ajay Srinivasan from Datar Cancer Genetics Limited, Nasik, India said, “It has been popularly believed that analyzing the molecular structure of cancer would yield definitive strategies and therapeutic direction for improved outcomes”
While platforms and solutions for molecular analysis of tumors have become ubiquitous, widespread adoption of treatment strategies based on evidence of molecular hallmarks appears to be stymied for want of definitive data and lack of demonstrable, quantifiable clinical benefits.
However, these studies were either based on univariate analysis of biomarkers and/or constrained in design by restricting inclusion to patients who were positive for a predefined molecular feature of the tumor.
Though there is evidence from prior trials that multi-drug combinations of cytotoxic and targeted agents may yield improved therapeutic benefit, fewer prior studies appear to have evaluated multi-drug combinations based on tumor molecular profiling.
As a consequence of these restrictions, the outcomes reported in prior precision medicine trials have either fallen short of expectations or have merely suggested equivocal to incremental improvements in efficacy , and have indicated the need for further evaluation of molecular guided therapy selection approach.
Accordingly, the authors designed the RESILIENT Study, where label-and organ-agnostic treatment strategies for patients with r/r m-cancers were based on an integrative, multi-analyte Encyclopedic Tumor Analysis which captures in depth information about the multi-layered tumor interactome.
The Srinivasan Research Team concluded, “The impact of previous treatments on the overall health of the patients, especially on the bone marrow reserve can impede compliance with ETA guided treatments. In the ITT population , among the 17 patients who were excluded prior to any follow-up, 6 patients were lost to follow-up due to inability to travel from other cities for treatment. Similarly, among the 17 patients who were excluded after the first evaluation, 12 patients were lost to follow-up for the same reason.”
###
Full text – http://www.
Correspondence to – Ajay Srinivasan – [email protected]
Keywords – precision oncology, encyclopedic tumor analysis, personalized cancer treatment, objective response rate, progression free survival
About Oncotarget
Oncotarget is a weekly, peer-reviewed, open access biomedical journal covering research on all aspects of oncology.
Oncotarget is published by Impact Journals LLC.
To learn more about Oncotarget, please visit http://www.
Media Contact
Ryan James Jessup
[email protected]
202-638-9720
Original Source
http://www.
Related Journal Article
http://dx.