Scientists report on the structural and molecular factors governing the stability of a protein complex involved in DNA repair pathways in cells
Credit: Tatsuya Nishino from Tokyo University of Science
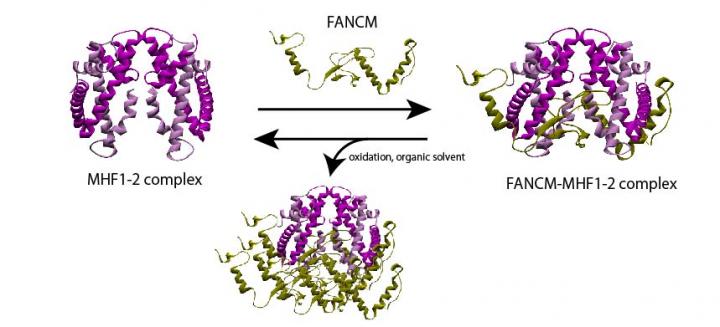
One of the most vital functions performed by the cells in our body is DNA repair, a task so crucial to our well-being that failing to execute it can lead to consequences as dreadful as cancer. The process of DNA repair involves a complex interplay between several gene pathways and proteins. One such pathway is the “Fanconi anemia (FA) pathway,” whose genes participate in DNA repair. FANCM, a component of this pathway, is tasked with the elimination of harmful DNA “inter-strand cross-links,” and interacts with another component called MHF in order to function. The importance of the FANCM-MHF complex is well-documented: its loss can result in chromosomal instabilities that can lead to diseases such as FA itself and cancer. However, little is known about its structure and the basis of its stability.
Against this backdrop, Associate Professor Tatsuya Nishino and his colleague Dr. Sho Ito from Tokyo University of Science decided to explore the crystalline structure of this intriguing complex using X-ray diffraction techniques. “DNA damage and chromosome segregation are mechanisms necessary for the maintenance and inheritance of genes possessed by all organisms. MHF (also known as CENP-SX) is an enigmatic complex that plays a role in DNA repair and chromosome segregation. We wanted to find out how it performs these two different functions in the hope that it might give us insights into novel phenomena,” explains Prof. Nishino. Their findings are published in Acta Crystallographica Section F: Structural Biology Communications.
The scientists prepared a recombinant version of the FANCM-MHF complex, consisting of FANCM from chickens and MHF1 and MHF2. They were able to purify three different types of protein crystals–tetrahedral, needle-shaped, and rod-shaped–from similar crystallization conditions. Surprisingly, upon determining the structure with X-ray crystallography, they found that two of the crystal forms (tetrahedral and needle-shaped) contained only the MHF complex without FANCM.
Intrigued by this discovery, the scientists used biochemical techniques to examine what caused the FANCM-MHF complex to disassemble. They attributed it to the presence of a compound called 2-methyl-2, 4-pentanediol (MPD), an organic solvent commonly used in crystallography, and exposure to an oxidizing environment.
But, how exactly does the dissociation happen? The scientists believe that this may have been caused partly by certain non-conserved amino acids in the chicken FANCM which causes the complex to aggregate with other FANCM-MHF complexes and disassemble. Additionally, they surmise that the small, flexible structure of MPD may have also allowed it to bind to and facilitate the release of FANCM, dismantling the complex.
The findings are extraordinary and can be used to improve the stability of the FANCM-MHF complex for future studies on its structure and function. Dr. Ito believes we have much to expect in the future from this complex. “A good understanding of this complex can help us treat cancer and genetic diseases, create artificial chromosomes, and even develop new biotechnological tools,” he speculates.
Thanks to Prof. Nishino and Dr. Ito’s efforts, we are already one step closer to that goal!
###
About The Tokyo University of Science
Tokyo University of Science (TUS) is a well-known and respected university, and the largest science-specialized private research university in Japan, with four campuses in central Tokyo and its suburbs and in Hokkaido. Established in 1881, the university has continually contributed to Japan’s development in science through inculcating the love for science in researchers, technicians, and educators.
With a mission of “Creating science and technology for the harmonious development of nature, human beings, and society”, TUS has undertaken a wide range of research from basic to applied science. TUS has embraced a multidisciplinary approach to research and undertaken intensive study in some of today’s most vital fields. TUS is a meritocracy where the best in science is recognized and nurtured. It is the only private university in Japan that has produced a Nobel Prize winner and the only private university in Asia to produce Nobel Prize winners within the natural sciences field.
Website: https:/
About Professor Tatsuya Nishino from Tokyo University of Science
Tatsuya Nishino is an associate professor at the Department of Biological Science and Technology at Tokyo University of Science, Japan. He received his masters and Doctor of Medicine (MD) degree from Osaka University, Japan in 1997 and 2001, respectively. His research area is molecular biology, where he works on structural biology of complexes involved in chromosome partitioning and chromosome segregation. He has 30 publications to his credit with over 1900 citations. Details about his research can be found here: https:/
Funding information
This work was supported by the collaboration project of the Platform Project for Supporting Drug Discovery and Life Science Research (Basis for Supporting Innovative Drug Discovery and Life Science Research; BINDS) from AMED (Grant No. 1739), NIG-JOINT (Grant Nos. 6A2017, 2A2018 and 85A2019), Cooperative Research Program of the Institute for Protein Research, Osaka University (Grant Nos. CR-17-05, CR-18-05 and CR-19-05), and Grants-in-Aid for Scientific Research from the Japanese Society for the Promotion of Science (Grant Nos. 16K07279 and 20K06512).
Media Contact
Tsutomu Shimizu
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




