Credit: University of Warwick
- Nitrous oxide is a greenhouse gas and ozone depleting substance.
- The ability to exploit this gas as a chemical reagent is an attractive prospect, both as an abundant feedstock and means to remediate the detrimental impact it has on the environment.
- Researchers at the University of Warwick have prepared transition metal compounds of nitrous oxide that provide a conceptional foundation for its application in new value-added chemical processes.
Nitrous oxide (N2O) is a potent atmospheric pollutant. Although naturally occurring, anthropogenic N2O emissions from intensive agricultural fertilisation, industrial processes, and combustion of fossil fuels and biomass are a major cause for concern. Researchers at the University of Warwick have isolated elusive transition metal compounds of N2O that provide clues into how it could be used in sustainable chemical technologies.
N2O is a powerful greenhouse gas, with a half life of 114 years in the atmosphere and global warming potential 300 times greater than carbon dioxide. It is also the dominant ozone depleting substance emitted in the 21st century.
As an abundant chemical feedstock, the use of N2O as a sustainable oxidant in synthetic organic chemistry is an attractive prospect, liberating environmentally benign dinitrogen (N2). Such reactions are encumbered by the robust triatomic formulation of this gas, typically requiring forcing reaction conditions that are energy intensive and undesirable from a remediation perspective. The development of mild and selective alternatives is a longstanding ambition of research scientists, but has been met with little success.
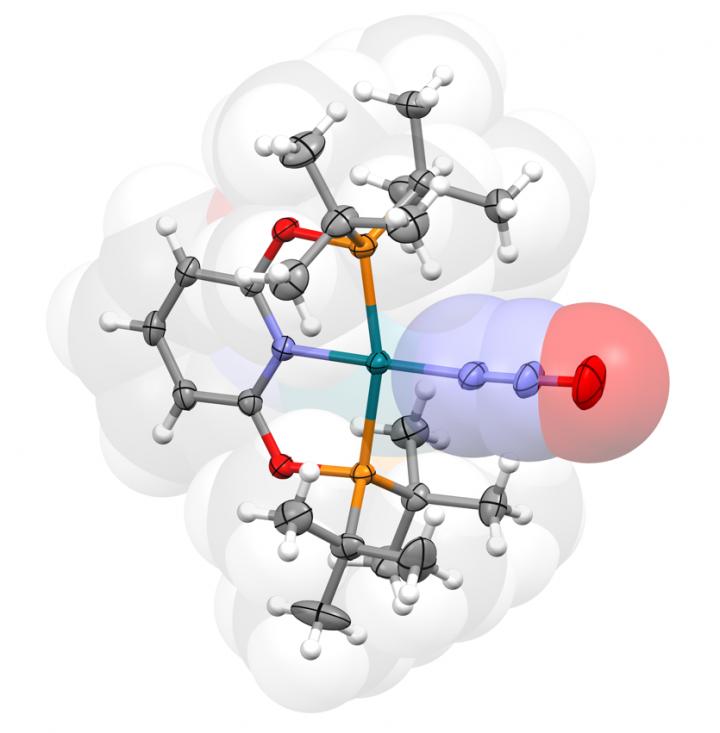
In their paper ‘Rhodium(I) Pincer Complexes of Nitrous Oxide’ published in the journal Angewandte Chemie, researchers from the University of Warwick’s Department of Chemistry have reported well-defined compounds of nitrous oxide that provide valuable insights into how this gas interacts with one of the most widely employed transition metals in organic synthesis.
The associated experimental data is the most comprehensive collected to date for any transition metal adduct, for which there are very few precedents. This work provides a fundamental reference point in the field and is likely to stimulate and guide future catalyst developments.
Dr Adrian Chaplin from the Department of Chemistry at the University of Warwick comments:
“Nitrous oxide is commonly known as laughing gas, but it’s environmental impact is certainly nothing to laugh about and often overlooked altogether. As a chemical reagent its potential has yet to be fully harnessed, and to do so a sustainable manner is formidable challenge for the scientific community.”
“In my team, we are trying to tackle this problem using a fundamental, bottom up, approach. The compounds that we have prepared represent the starting point of our journey, but the associated experimental data seems to be guiding us in the right direction and we are looking forward to where it takes us.”
###
NOTES TO EDITORS
Paper available to view at: http://doi.
High-res images available credit to the University of Warwick:
https:/
For further information please contact:
Alice Scott
Media Relations Manager – Science
University of Warwick
Tel: +44 (0) 2476 574 255 or +44 (0) 7920 531 221
E-mail: [email protected]
Media Contact
Alice Scott
[email protected]
Original Source
https:/
Related Journal Article
http://dx.




