Revolutionary lung valve is the first FDA-approved device to help emphysema patients breathe easier without major surgery
Credit: Pulmonx Corporation
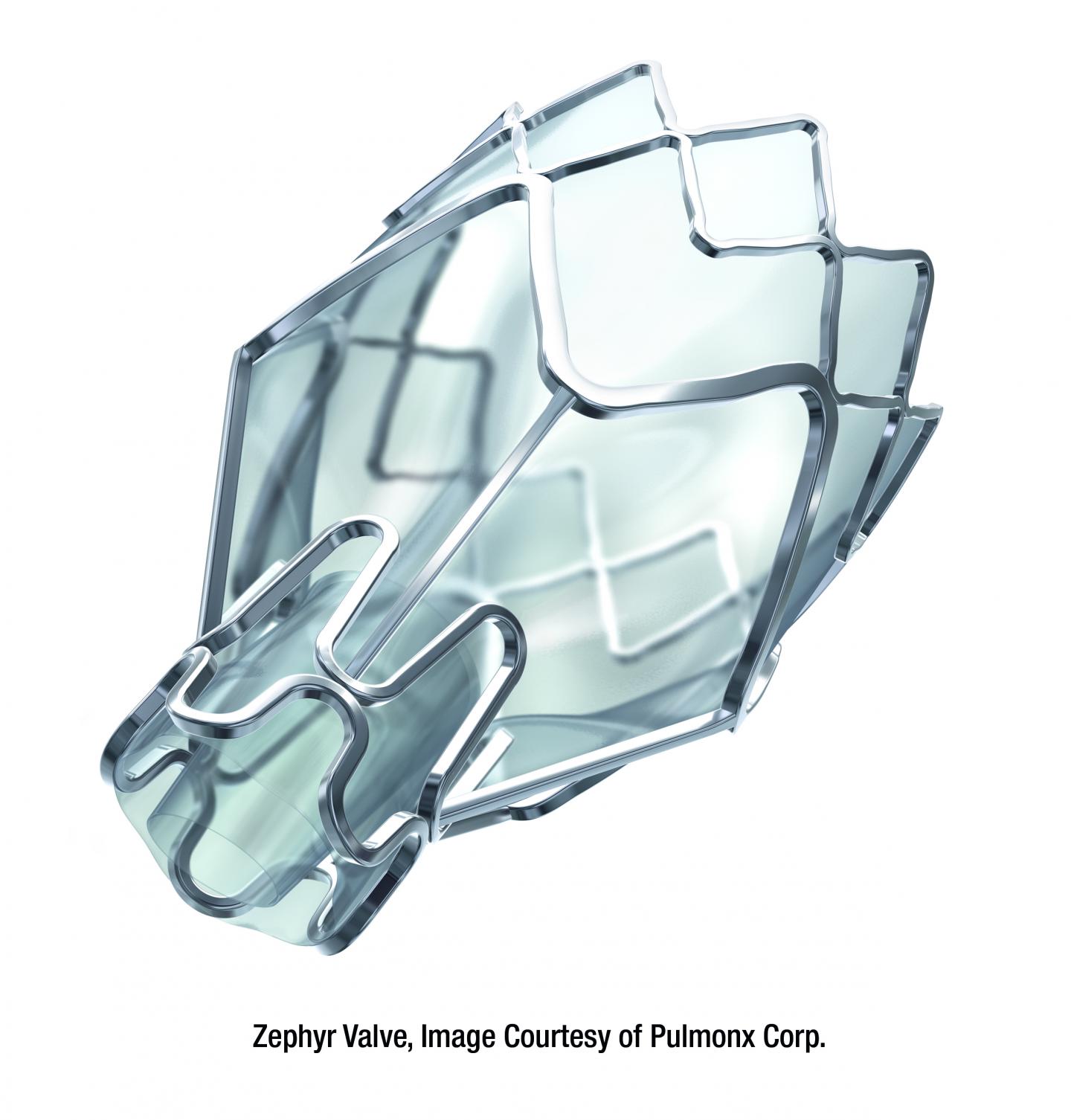
Rancho Mirage, CA (April 24, 2019) Eisenhower Health is the first hospital in Southern California to offer a new lung valve treatment for patients with severe COPD/emphysema. Recently approved by the FDA under their “Breakthrough Devices” status, the Zephyr Endobronchial Valve treatment represents a major advancement because it is the first minimally invasive procedure to help emphysema sufferers breathe easier without major surgery. Done through a simple bronchoscopy, the valves improve patients’ quality of life by allowing them to breathe easier, be less short of breath, and be more active and energetic.
“We are very excited to offer this new treatment option,” says Justin Thomas, MD, who is Board Certified in Internal Medicine, Pulmonary Disease, Critical Care Medicine, and Interventional Pulmonary Medicine and the Director of the Bronchoscopy Laboratory, Eisenhower Health. “Emphysema patients are often in poor physical condition, struggling with each breath despite medication therapy. Before the Zephyr Valves the only options for relief were highly invasive treatments including lung surgeries. Being able to offer this minimally invasive procedure has the potential to improve the quality of life for many emphysema sufferers in the Coachella Valley and beyond.”
Emphysema is a progressive and life-threatening lung disease, and a severe form of chronic pulmonary obstructive disease (COPD). There is no cure and patients live with severe shortness of breath that keeps them from doing simple daily activities like walking or taking a shower without pausing to catch their breath or resting. This extreme shortness of breath is caused when air becomes trapped in parts of the lung that are damaged by the disease. This trapped air causes the damaged areas of the lungs to get larger which puts pressure on the healthy parts of the lungs and diaphragm.
During this one-time, short procedure, on average, a physician places four tiny valves in the airways to block off the damage areas of the lungs so air no longer gets trapped there. This allows the healthier parts of the lungs to expand and relieves the pressure on the diaphragm, which decreases shortness of breath and makes breathing easier.
The Zephyr® Valves were fast-tracked through the FDA’s “Breakthrough Device” status because they “offer bronchoscopic lung volume reduction without surgery and its associated risks.” The FDA’s approval was based on the results of four randomized controlled clinical trials, including the U.S. approval study, LIBERATE. Data from the study showed that implantation of the Zephyr Valves successfully reduced shortness of breath while improving lung function, exercise capacity, and quality of life.1 These benefits lasted at least one-year post-treatment for patients with severe emphysema.
The Zephyr Valves were approved by the FDA in July 2018. Since 2007, more than 15,000 patients have been treated with The Zephyr Valve worldwide. Zephyr Valve treatment is included in emphysema treatment recommendations issued by leading health organizations worldwide, including the Global Initiative for Chronic Obstructive Lung Disease (GOLD) and the UK’s National Institute for Health and Care Excellence (NICE).
Eisenhower Health is a not-for-profit, comprehensive health care institution that includes the 463-bed Eisenhower Hospital, the Barbara Sinatra Children’s Center at Eisenhower and the Annenberg Center for Health Sciences at Eisenhower. The Betty Ford Center is also located on the Eisenhower campus. Eisenhower is renowned for its Centers of Excellence in Orthopedics, Cardiovascular, Neuroscience and Oncology. Situated on 130 acres in Rancho Mirage, and with outpatient clinics across the valley, Eisenhower Health has provided a full range of quality medical and educational services for more than 45 years for residents and visitors to the greater Coachella Valley. Eisenhower has earned ANCC Magnet Recognition® for professionalism in nursing and excellence in patient care. The first accredited teaching hospital in the valley, Eisenhower trains physician residents in both Internal Medicine and Family Medicine. For more information, visit EisenhowerHealth.org or follow Eisenhower Health on social media.
###
For on camera or phone interviews, contact Lee Rice | Media Coordinator and Communications Specialist at 310. 210. 6192.
1. Criner G, Sue R, Wright S, Dransfield M, Rivas-Perez H, Wiese T et al. A multicenter RCT of Zephyr® Endobronchial Valve Treatment in heterogeneous emphysema (LIBERATE). Am J Resp Crit Care Med. Published online May 22, 2018. https:/
Media Contact
Meghan K Oreste
[email protected]




