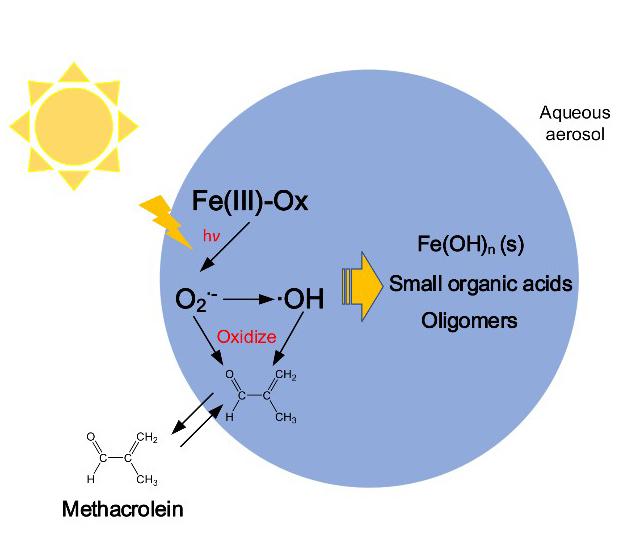
The Fenton reaction is a chemical transition involving hydrogen peroxide (H2O2) and the ferrous (iron) ion, which acts as a catalyst. This process is used to destroy hazardous contaminants in wastewater through oxidation. In the atmosphere, a similar reaction, or “Fenton-like” reaction, occurs continuously with ferric oxalate([Fe(III)(C2O4)3]3-) and aerosols suspended in the air. This is the most frequent chemical reaction that occurs in the atmosphere. A particle’s ability to oxidize is directly related to its phase, either gaseous or aqueous, which has an important impact on secondary organic aerosol (SOA) formation. Therefore, research is necessary not only to evaluate the contribution of this Fenton-like reaction to atmospheric oxidation, but also to improve the consistency of model-simulated and field-observed SOA budgets.
“It is generally believed that the contribution of the Fenton reaction to atmospheric oxidation comes from the generation of hydroxyl radicals.” said Prof. Wenbo Dong from the Department of Environmental Science & Engineering, Fudan University. “Scientists have not frequently addressed the role of superoxide radicals, which is generally regarded as the source of hydrogen peroxide and hydroxyl radicals.”
Methacrolein (CH2=C(CH3)CHO) is the primary oxidation product of isoprene(CH2=C(CH3)CH=CH2), which is the most abundant biological volatile organic compound (VOC) in the atmosphere. It can directly react with superoxide radicals to generate SOAs. While this is a common reaction, this process shows that other paths to VOC oxidation exist.
“Previous studies believed that superoxide radicals do not react with most organic compounds.” remarked Prof. Dong.
Some VOCs in the atmosphere may react with superoxide radicals just like methacrolein. However, SOA production potential from any VOC with attendant superoxide radicals and hydroxyl radicals is distinct from the methacrolein reaction. Researchers focused on the oxidation process of organic pollutants caused by these free radicals. They found that the oxidation process is related to the reaction mechanism of the organic matter accompanying these free radicals.
Previous studies have shown that the change in aqueous aerosol absorbance is attributed to the formation of brown carbon. However, in the case of photo-oxidation of methacrolein with ferric oxalate, Prof. Dong’s research group noticed a substantial increase in aerosol absorbance without formation of brown carbon. Further analysis is provided in their research article titled “Photooxidation of Methacrolein in Fe(III)-Oxalate Aqueous System and Its Atmospheric Implication” published in Advances in Atmospheric Sciences.
“When the Fenton-like reaction with a high concentration of iron occurs, the absorbance of the solution will change significantly, with the solution turning yellow.” said Prof. Dong. “This may not be the only situation with methacrolein, as it can come up with the Fenton-like reaction of other organic compounds.”
Prof. Dong continued, “The formation of insoluble or colloidal iron hydroxide increases the absorbance of atmospheric aerosols, affecting the radiative forcing, which has been overlooked for a long time.”
###
Media Contact
Ms. Zheng Lin
[email protected]
Related Journal Article
http://dx.