Credit: © Cristina Mayor-Ruiz / CeMM
Despite enormous efforts to advance traditional pharmacology approaches, more than three quarters of all human proteins remain beyond the reach of therapeutic development. Targeted protein degradation (TPD) is a novel approach that could overcome this and other limitations, and thus represents a promising therapeutic strategy. TPD is based on small molecules, generally called”degraders”, which can eliminate disease-causing proteins by causing their destabilization. Mechanistically, these degrader drugs repurpose the cellular protein quality control system, tweaking it to recognize and eliminate harmful proteins. In detail, they re-direct members of the protein family of E3 ubiquitin ligases (E3s) towards the disease-causing target protein. This leads to a “molecular earmarking” of the harmful protein via a process called “ubiquitination”. Subsequently, the ubiquitinated protein is recognized and degraded by the molecular machine called the proteasome, which serves as the cellular garbage disposal system.
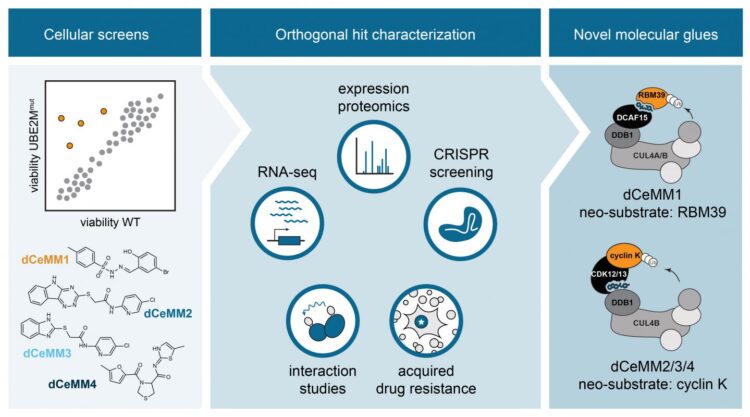
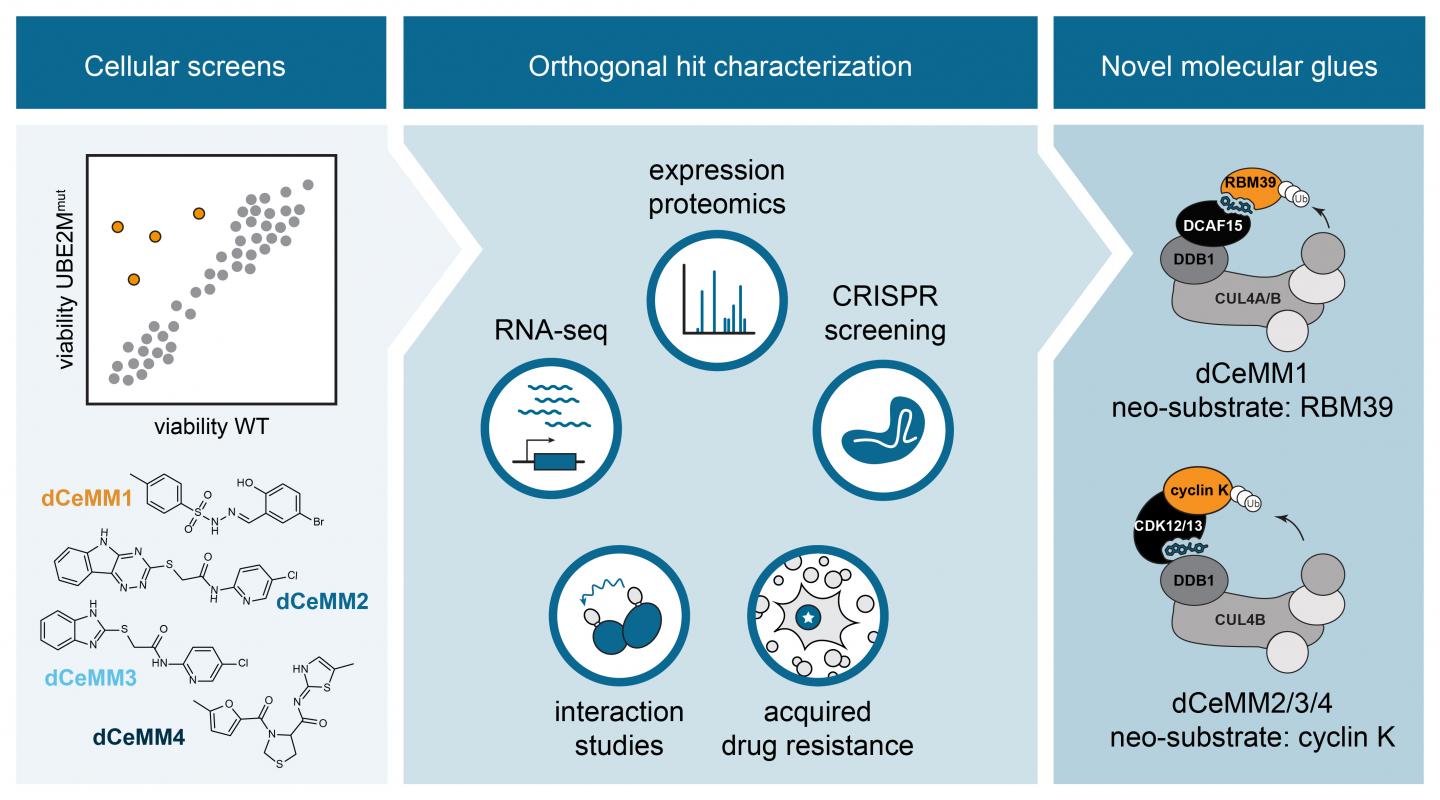
In this study, CeMM researchers turned their focus to a subset of degraders called “molecular glue degraders”. This class of seemingly rare small molecules that has been shown to induce the degradation of target proteins that could not be blocked via ways of traditional pharmacology. Consequently, these proteins had been termed “undruggable”. The best characterized examples are the clinically approved thalidomide analogs, effective for the treatment of different blood cancers. Unfortunately, the discovery of the few described molecular glue degraders has historically been a process entirely driven by serendipity and no rational discovery strategies existed.
To overcome this limitation, Georg Winter’s group at CeMM set out to innovate a scalable strategy towards the discovery of novel molecular glue degraders via phenotypic chemical screening. To this end, first author and CeMM postdoctoral fellow Cristina Mayor-Ruiz and colleagues engineered cellular systems widely impaired in E3 activity. Differential viability between these models and E3-proficient cells was used to identify compounds that depend on active E3s, and therefore, potential molecular glue degraders. Researchers integrated functional genomics with proteomics and drug-interaction strategies, to characterize the most promising compounds. They validated the approach by discovering a new RBM39 molecular glue degrader, structurally similar to others previously described. Importantly, they discovered a set of novel molecular glues that induce the degradation of the protein cyclin K, known to be essential in many different cancer types. Interestingly, these novel cyclin K degraders function via an unprecedented molecular mechanism of action that involves the E3 CUL4B:DDB1 and that has never been therapeutically explored before.
This study, performed in close collaboration with CeMM PI Stefan Kubicek, thus provides the first framework towards the discovery of molecular glue degraders that can be highly scaled, but also strongly diversified. “I truly believe that we are only scratching the surface of possibilities. This study is chapter one of many chapters to follow. We will see a revolution in the way researchers perceive and execute therapeutic strategies for previously incurable diseases by crafting glue degrader strategies that will enable them to eliminate therapeutic targets that could not be explored with traditional pharmacologic approaches”, says CeMM PI and last author of the study Georg Winter.
###
The study “Rational discovery of molecular glue degraders via scalable chemical profiling” was published in Nature Chemical Biology on 3 August 2020. DOI: 10.1038/s41589-020-0594-x
Authors:
Cristina Mayor-Ruiz, Sophie Bauer*, Matthias Brand*, Zuzanna Kozicka, Marton Siklos, Hana Imrichova, Ines Kaltheuner, Elisa Hahn, Kristina Seiler, Anna Koren, Georg Petzold, Michaela Fellner, Christoph Bock, Andre. Mueller, Johannes Zuber, Matthias Geyer, Nicolas H. Thomä, Stefan Kubicek, Georg E. Winter | *equal contribution
Funding:
The study was supported by the Austrian Academy of Sciences, the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (Starting grant agreement No 851478) and the Austrian Science Fund (FWF project number P32125-B and P30271-B28). C. Mayor-Ruiz was supported by an individual Marie Sklodowska-Curie postdoctoral fellowship (grant agreement No 796010).
Georg Winter obtained his PhD from the Medical University of Vienna, working on elucidating the mechanism of action of anti-neoplastic drugs under the supervision of Prof. Giulio Superti-Furga at CeMM. He specialized in proteomics as well as chemical genetic approaches to identifying drug resistance mechanisms and on mechanistically elucidating synergistic drug combinations. He continued his training in chemical biology, working as a postdoctoral fellow with Dr. James Bradner at the Dana Farber Cancer Institute/Harvard Medical School. There, he innovated a generalizable pharmacological solution to in vivo target protein degradation and applied this strategy to the study of leukemic gene regulation. Georg Winter was recruited as a CeMM Principal Investigator in June 2016. His lab develops and applies methods for target protein degradation with the ultimate goal of understanding and disrupting oncogenic transcriptional circuits. To that end, the Winter laboratory combines phenotypic drug screens, chemical genetics and drug-target identification approaches with holistic measurements of global gene activity and genome structure. The ultimate goal of the research conducted in the Winter laboratory is to connect basic research in gene regulation and the ubiquitin-proteasome system with functional genomics and chemical probe development to develop novel and personalized therapeutic paradigms.
The mission of CeMM Research Center for Molecular Medicine of the Austrian Academy of Sciences is to achieve maximum scientific innovation in molecular medicine to improve healthcare. At CeMM, an international and creative team of scientists and medical doctors pursues free-minded basic life science research in a large and vibrant hospital environment of outstanding medical tradition and practice. CeMM’s research is based on post-genomic technologies and focuses on societally important diseases, such as immune disorders and infections, cancer and metabolic disorders. CeMM operates in a unique mode of super-cooperation, connecting biology with medicine, experiments with computation, discovery with translation, and science with society and the arts. The goal of CeMM is to pioneer the science that nurtures the precise, personalized, predictive and preventive medicine of the future. CeMM trains a modern blend of biomedical scientists and is located at the campus of the General Hospital and the Medical University of Vienna. http://www.
Media Contact
Laura Alvarez
[email protected]
Original Source
https:/
Related Journal Article
http://dx.