Drug targets with human genetic evidence are more likely to be clinically translatable and thus enter phase II/III clinical trials or be approved for marketing more quickly, which will likely significantly reduce the cost of drug development and drive the rapid development of the pharmaceutical industry. Currently, genetic data and technologies such as genome-wide association studies (GWAS), whole-exome sequencing (WES), and whole-genome sequencing (WGS) have identified and validated many potential molecular targets related to diseases. Although most LOF mutations result in increased disease risk, there are still some LOF benign mutations that can produce natural disease protection, providing critical support for targeted drug development.
Credit: Not applicable
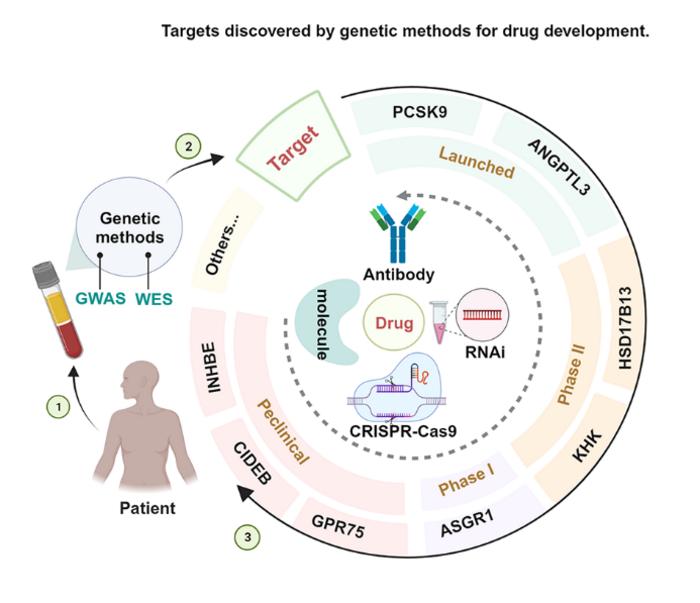
Drug targets with human genetic evidence are more likely to be clinically translatable and thus enter phase II/III clinical trials or be approved for marketing more quickly, which will likely significantly reduce the cost of drug development and drive the rapid development of the pharmaceutical industry. Currently, genetic data and technologies such as genome-wide association studies (GWAS), whole-exome sequencing (WES), and whole-genome sequencing (WGS) have identified and validated many potential molecular targets related to diseases. Although most LOF mutations result in increased disease risk, there are still some LOF benign mutations that can produce natural disease protection, providing critical support for targeted drug development.
The review briefly explains the mechanism of action of the eight beneficial LOF mutation targets and lists the marketed and investigational drugs.PCSK9 regulates LDL-C levels through the LDLR, and two monoclonal antibodies (alirocumab, evolocumab) and an siRNA (inclisiran) are currently approved and marketed for the treatment of patients with hyperlipidemia. ANGPTL3 regulates LDL-C and TG levels by inhibiting lipoprotein lipase (LPL) and endothelial lipase (EL) activity, and a monoclonal antibody, evinacumab, is currently approved and marketed for the treatment of patients with HoFH. ASGR1 has great potential as a target for regulating cholesterol efflux and fatty acid synthesis for lipid-lowering drug development, and drugs and technologies targeting ASGR1 are currently in the development stage. HSD17B13 is involved in hepatic lipid metabolism and is one of the emerging potential targets for NAFLD/NASH, with three drugs currently in Phase II clinical stage (ARO-HSD, ALN-HSD and FOR-6219). KHK is one of the rate-limiting enzymes of fructose metabolism and has therapeutic implications for NAFLD/NASH, T2D and other fructose-mediated metabolic diseases, with two drugs currently in Phase II clinical stage (ALN-KHK and PF-06835919). CIDEB plays an important role in the maintenance of systemic lipid homeostasis and energy metabolism, and blocking CIDEB expression may help to prevent or treat NASH and related disorders, but there are no drugs for this purpose that are currently in clinical trials, although Regeneron has partnered with Alnylam to develop an siRNA therapeutic candidate that silences the CIDEB gene. GPR75 LOF has been linked to the prevention of obesity and is expected to be a new potential target for the treatment of obesity and related complications, and Regenerative Elements has now partnered with AstraZeneca to develop a small molecule therapeutic against GPR75. INHBE LOF mutations help reduce the prevalence of abdominal obesity, metabolic syndrome, coronary heart disease, and T2D, and Alnylam has utilised its liver KARIA platform for nucleic acid drug development targeting INHBE.
Based on a thorough understanding and summary of beneficial LOF mutation targets, new insights are provided for drug development that mimic the natural disease-protective effects of rare LOF variants.
Journal
MedComm
DOI
10.1002/mco2.481
Method of Research
Systematic review
Subject of Research
Not applicable
Article Title
Drug development advances in human genetics-based targets
Article Publication Date
9-Feb-2024
COI Statement
The authors declare that they have no conflict of interest.




