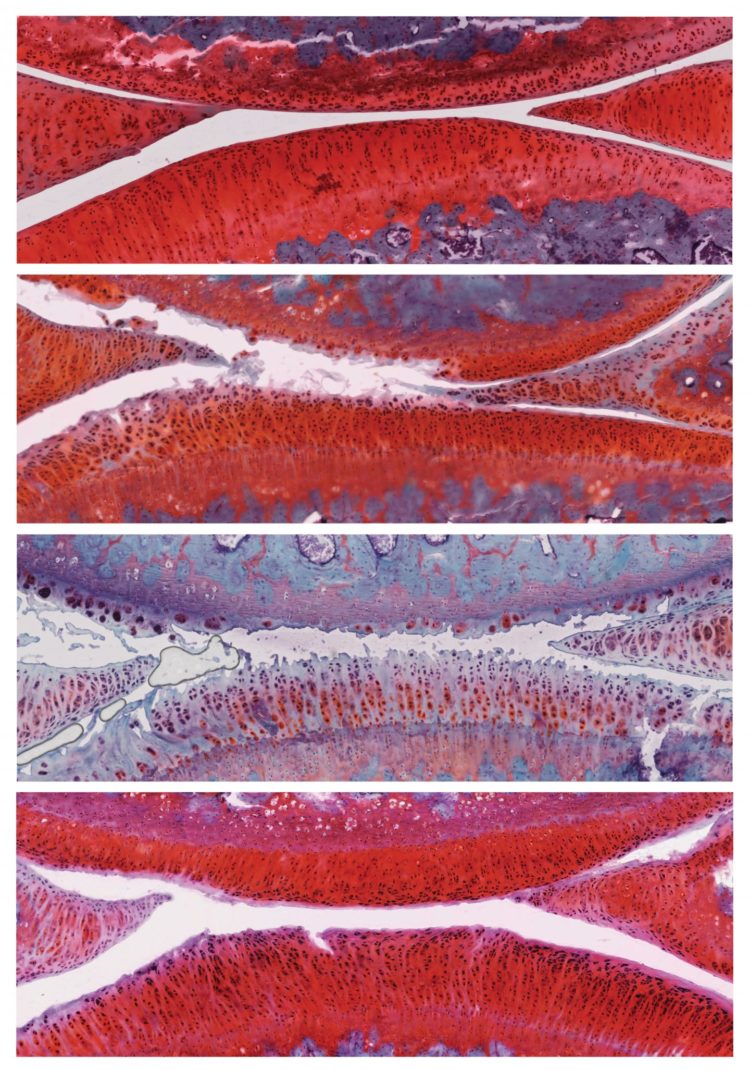
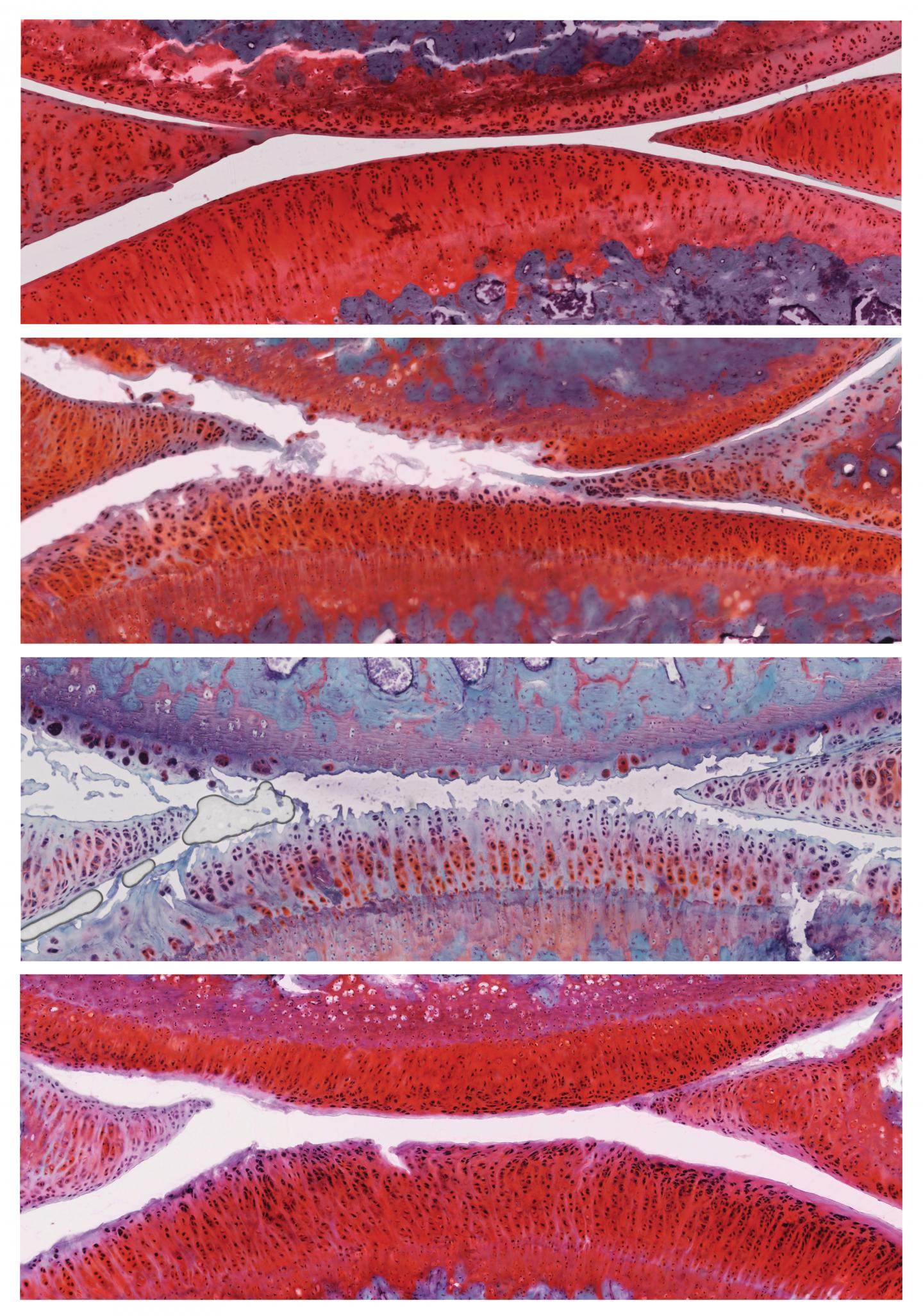
A combination of 2 previously studied osteoarthritis drugs work better than either drug alone, Salk researchers discovered
Credit: Salk Institute
LA JOLLA–(January 21, 2020) People with osteoarthritis, or “wear and tear” arthritis, have limited treatment options: pain relievers or joint replacement surgery. Now, Salk researchers have discovered that a powerful combination of two experimental drugs reverses the cellular and molecular signs of osteoarthritis in rats as well as in isolated human cartilage cells. Their results were published in the journal Protein & Cell on January 16, 2020.
“What’s really exciting is that this is potentially a therapy that can be translated to the clinic quite easily,” says Juan Carlos Izpisua Belmonte, lead author and a professor in Salk’s Gene Expression Laboratory. “We are excited to continue refining this promising combination therapy for human use.”
Affecting 30 million adults, osteoarthritis is the most common joint disorder in the United States and its prevalence is expected to rise in coming years due to the aging population and increasing rate of obesity. The disease is caused by gradual changes to cartilage that cushions bones and joints. During aging and repetitive stress, molecules and genes in the cells of this articular cartilage change, eventually leading to the breakdown of the cartilage and the overgrowth of underlying bone, causing chronic pain and stiffness.
Previous research had pinpointed two molecules, alpha-KLOTHO and TGF beta receptor 2 (TGFβR2), as potential drugs to treat osteoarthritis. αKLOTHO acts on the mesh of molecules surrounding articular cartilage cells, keeping this extra-cellular matrix from degrading. TGFβR2 acts more directly on cartilage cells, stimulating their proliferation and preventing their breakdown.
While each drug alone had only moderately curbed osteoarthritis in animal models of the disease, Izpisua Belmonte and his colleagues wondered if the two drugs would act more effectively in concert.
“We thought that by mixing these two molecules that work in different ways, maybe we could make something better,” says Paloma Martinez-Redondo, a Salk research associate and first author of the new study.
The researchers treated young, otherwise healthy rats with osteoarthritis with viral particles containing the DNA instructions for making αKLOTHO and TGFβR2.
Six weeks after the treatment, rats that had received control particles had more severe osteoarthritis in their knees, with the disease progressing from stage 2 to stage 4. However, rats that had received particles containing αKLOTHO and TGFβR2 DNA showed recovery of their cartilage: the cartilage was thicker, fewer cells were dying, and actively proliferating cells were present. These animals’ disease improved from stage 2 to stage 1, a mild form of osteoarthritis, and no negative side effects were observed.
“From the very first time we tested this drug combination on just a few animals, we saw a huge improvement,” says Martinez-Redondo. “We kept checking more animals and seeing the same encouraging results.”
Further experiments revealed 136 genes that were more active and 18 genes that were less active in the cartilage cells of treated rats compared to control rats. Among those were genes involved in inflammation and immune responses, suggesting some pathways by which the combination treatment works.
To test the applicability of the drug combination to humans, the team treated isolated human articular cartilage cells with αKLOTHO and TGFβR2. Levels of molecules involved in cell proliferation, extra-cellular matrix formation and cartilage cell identity all increased.
“That’s not the same as showing how these drugs affect the knee joint in humans, but we think it’s a good sign that this could potentially work for patients,” says Martinez-Redondo.
The research team plans to develop the treatment further, including investigating whether soluble molecules of the αKLOTHO and TGFβR2 proteins can be taken directly, rather than administered through viral particles. They also will study whether the combination of drugs can prevent the development of osteoarthritis before symptoms develop.
“We think that this could be a viable treatment for osteoarthritis in humans,” says Pedro Guillen, director of the Clinica CEMTRO and co-corresponding author.
###
Other authors were Isabel Guillen-Guillen, Chao Wang, Javier Prieto, Masakazu Kurita, Fumiyuki Hatanaka, Cuiqing Zhong, Reyna Hernandez-Benitez, Tomoaki Hishida, Takashi Lezaki, Akihisa Sakamoto, Amy Nemeth, Yuriko Hishida, Concepcion Rodriguez Esteban, Kensaku Shojima, Pradeep Reddy, Ling Huang and Maxim Shokhirev of Salk; Noah Davidson and George Church of Harvard University; Estrella Nuñez-Delicado of Universidad Católica San Antonio de Murcia; Josep Campistol of Hospital Clinic of Barcelona; Isabel Guillen-Vicente, Elena Rodriguez-Iñigo, Juan Manuel Lopez-Alcorocho, Marta Guillen-Vicente and Pedro Guillen-Garcia of Clinica CEMTRO; and Guang-Hui Liu of Chinese Academy of Sciences.
About the Salk Institute for Biological Studies:
Every cure has a starting point. The Salk Institute embodies Jonas Salk’s mission to dare to make dreams into reality. Its internationally renowned and award-winning scientists explore the very foundations of life, seeking new understandings in neuroscience, genetics, immunology, plant biology and more. The Institute is an independent nonprofit organization and architectural landmark: small by choice, intimate by nature and fearless in the face of any challenge. Be it cancer or Alzheimer’s, aging or diabetes, Salk is where cures begin. Learn more at: salk.edu.
Media Contact
Salk Communications
[email protected]
858-453-4100
Related Journal Article
http://dx.