How do signals from outside the cell cause a response inside it? Such outside signals could be hormones or neurotransmitters. To notice them, the cell’s surface possesses receptors. One of the key classes of such receptors are so-called G protein-coupled receptors, or in short GPCRs. They are proteins placed on the cell’s membrane. Once an outside signal activates them, they trigger processes inside the cell with far-reaching impacts on cell growth, migration, and metabolism as well as cell-to-cell communication. This group of more than 800 receptors also plays a vital role in many diseases. Therefore, GPCRs have become important targets of drugs. In fact, 35% of all drugs approved by the US Food and Drug Administration (FDA) target GPCRs, accounting for an annual market of estimated 180 billion US dollars.
Credit: © Rouven Schulz / ISTA
How do signals from outside the cell cause a response inside it? Such outside signals could be hormones or neurotransmitters. To notice them, the cell’s surface possesses receptors. One of the key classes of such receptors are so-called G protein-coupled receptors, or in short GPCRs. They are proteins placed on the cell’s membrane. Once an outside signal activates them, they trigger processes inside the cell with far-reaching impacts on cell growth, migration, and metabolism as well as cell-to-cell communication. This group of more than 800 receptors also plays a vital role in many diseases. Therefore, GPCRs have become important targets of drugs. In fact, 35% of all drugs approved by the US Food and Drug Administration (FDA) target GPCRs, accounting for an annual market of estimated 180 billion US dollars.
While so important, major challenges exist in investigating GPCRs. First, many of the substances that bind to them, so-called ligands, are unknown. Second, even the known ligands may have poor availability in the organism or cause unwanted side-effects. And third, it is difficult to pinpoint responses, when the same receptor is present on different cells within the body, meaning that in immune cells the same GPCR may modulate inflammation, while in bronchial tissue it may relax muscles. “We wanted to overcome these limitations. So we developed an accessible and effective toolbox for everyone, who studies GPCRs,” says Rouven Schulz, doctoral student at the Institute of Science and Technology Austria (ISTA) and lead-author of the study published in Nature Communications.
Mimicking a receptor
In simplified terms, Schulz and his colleagues engineered artificial replicas of GPCRs. The replicas are ignorant to the original outside signals, but can be activated by an unrelated drug, which is precisely designed to only signal to this receptor and therefore easily controllable. The known framework of creating Designer Receptors Exclusively Activated by Designer Drugs (DREADDs) allowed the authors to produce chimeras of virtually any GPCR. For the proof of principle, they chose β2AR – a receptor of broad biological importance, also in humans. The replica of β2AR is selectively activated by a known drug called clozapine-N-oxide. “Once we had a prototypic chimera of β2AR, we could successfully reproduce many of the original’s behavior in the living organism. Amazingly, the chimera mimics the receptor accurately across many functions,” explains Schulz.
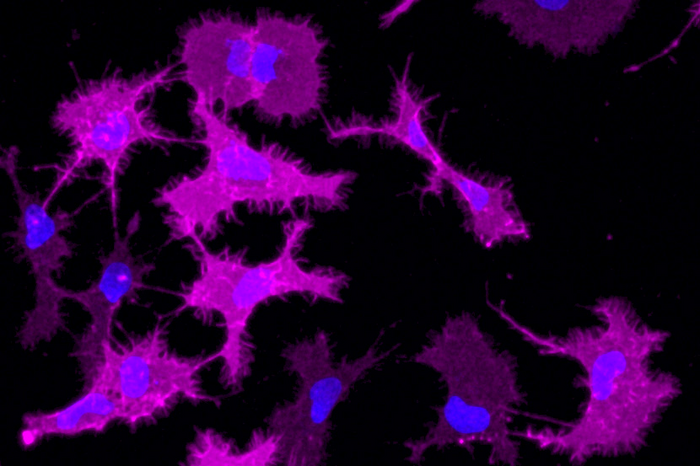
In the last stage, the researchers applied the method to microglia cells. These immune cells are key cells in the maintenance of the brain and the overall central nervous system. They constantly scavenge for infectious agents as well as damaged or non-functional neuronal connections. “GPCRs, specifically β2AR, are critical for these functions of microglia cells. Equipped with our chimera receptor of β2AR, the microglia cells replicated the process of driving inflammation in the nervous system.”
The bigger picture
Looking closely at GPCRs in microglia cells and overall immune cells, one exhibits a broad variety of them. Yet, at this point, nobody knows why organisms and humans have such a diversity of these receptors. Oftentimes, GPCRs are categorized in the main ways of how they forward signals, the so-called signaling pathways. “But we see in our data that there are subtle differences between receptors even when they drive the same overall pathway,” says Assistant Professor Sandra Siegert. “That suggests fine-tuned properties that transcend these standard canonical pathways. Now, we have a possibility to look into these details.” Currently, the group brings the method to the human regime using stem cells. By this, they aim to investigate how various kinds of inflammatory characteristics change upon GPCR stimulation.
Publication:
Rouven Schulz, Medina Korkut-Demirbaş, Gloria Colombo, Alessandro Venturino, and Sandra Siegert. 2022. Chimeric GPCRs mimic distinct signaling pathways and modulate microglia responses. Nature Communications. DOI: 10.1038/s41467-022-32390-1.
Funding information:
This research was supported by a DOC Fellowship by the Austrian Academy of Sciences.
Information on animal studies:
The validation of the DREADD-β2AR prototype is only possible by inserting them into living animals, since microglia change entirely in their genetic, molecular, and functional signature outside this environment. No other methods, such as in silico models, can serve as alternative. The animals are raised, kept, and treated according to the strict regulations of Austrian law. All animal procedures are approved by the Federal Ministry of Education, Science and Research.
Journal
Nature Communications
DOI
10.1038/s41467-022-32390-1




