New experimental findings cast in doubt 90-year-old theoretical model of the transition state in chemical reactions
Credit: Illustration: Lydie Leung
TORONTO, ON – Research by a team of chemists at the University of Toronto, led by Nobel Prize-winning researcher John Polanyi, is shedding new light on the behaviour of molecules as they collide and exchange atoms during chemical reaction. The discovery casts doubt on a 90-year old theoretical model of the behavior of the “transition state”, intermediate between reagents and products in chemical reactions, opening a new area of research.
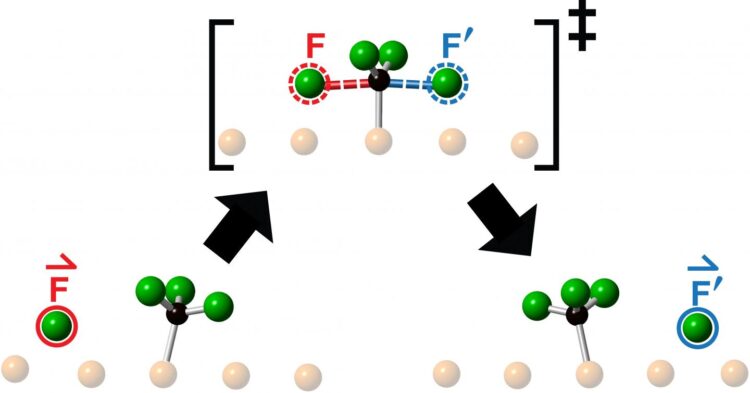
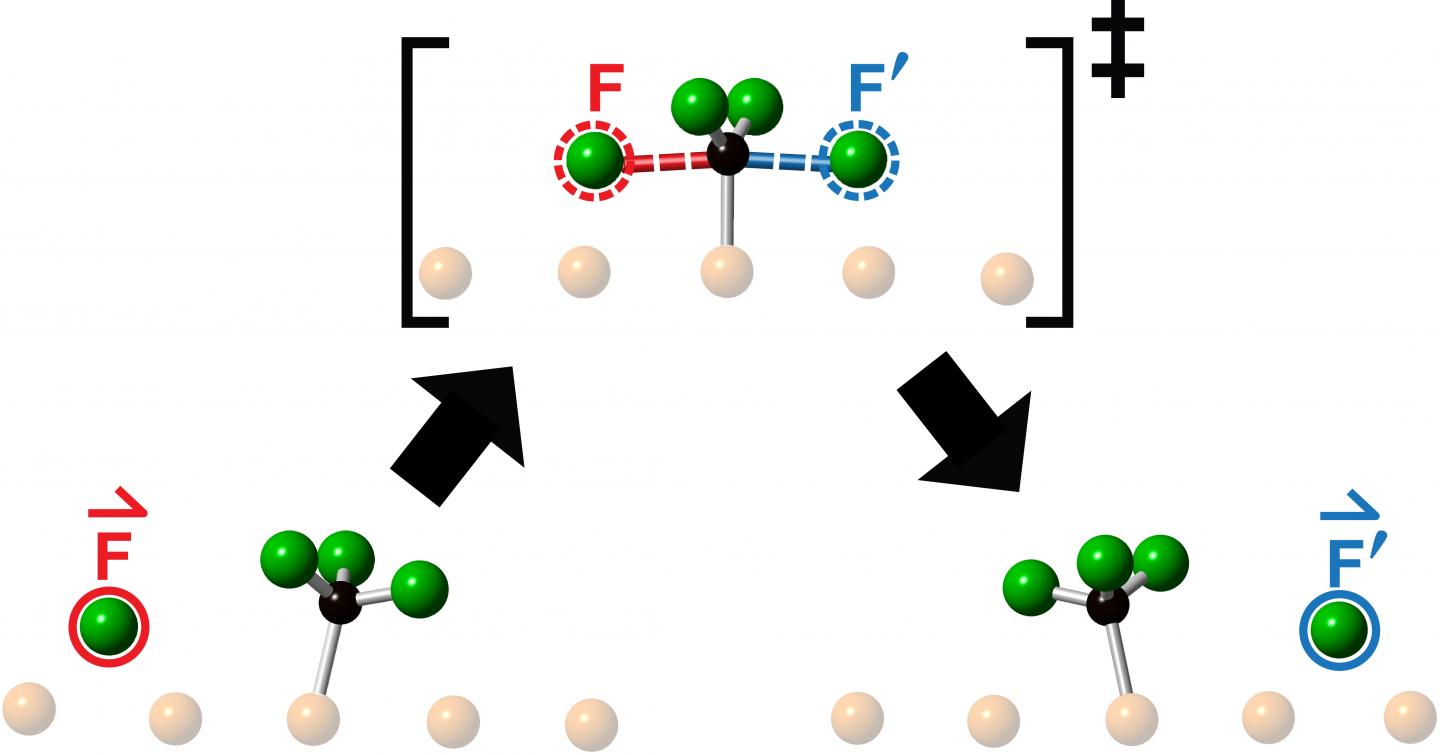
The researchers studied collisions obtained by launching a fluorine atom at the centre of a fluoromethyl molecule – made up of one carbon atom and three fluorine atoms – and observed the resulting reaction using Scanning Tunneling Microscopy. What they saw following each collision was the ejection of a new fluorine atom moving collinearly along the continuation of the direction-of-approach of the incoming fluorine atom.
“Chemists toss molecules at other molecules all the time to see what happens or in hopes of making something new,” says Polanyi, University Professor in the Department of Chemistry in the Faculty of Arts & Science at U of T and senior author of a study published this month in Communications Chemistry. “We found that aiming a reagent molecule at the centre of a target molecule, restricts the motion of the emerging product to a single line, as if the product had been directly ‘knocked-on’. The surprising observation that the reaction product emerges in a straight line, moving in the same direction as the incoming reagent atom, suggests that the motions that lead to reaction resemble simple onward transfer of momentum.
“The conservation of linear momentum we observe here suggests a short-lived “transition state”, rather than the previous view that there is sufficient time for randomization of motion. Newton would, I think, have been pleased that nature permits a simple knock-on event to describe something as complex as a chemical reaction,” says Polanyi.
The team, which included senior research associate Lydie Leung, graduate student Matthew Timm and PhD graduate Kelvin Anggara, had previously established the means to control whether a molecule launched towards another either collides head-on with its target or misses by a chosen amount – a quantity known as the impact parameter. The higher the impact parameter, the greater the distance by which the incoming molecule misses the target molecule. For the new work, the researchers employed an impact parameter of zero to give head-on collision.
“We call this new type of one-dimensional chemical reaction ‘knock-on’, since we find that the product is knocked-on along the continuation of the direction of reagent approach,” says Polanyi. “The motions resemble the knock-on of the steel balls of a Newton’s cradle. The steel balls of the cradle don’t pass through one another, but efficiently transfer momentum along a single line.
“Similarly, our knock-on reactions transfer energy along rows of molecules, thereby favouring a chain-reaction. This conservation of reaction energy in knock-on chemistry could be useful as the world moves toward energy conservation. For now, it serves as an example of chemical reaction at its simplest.”
It has been known for well over a century that there is an energy barrier that chemical reagents must cross on their way to forming reaction products. An energized transition state exists briefly at the top of the barrier in a critical configuration – no transition state, no reaction.
Polanyi says the observation of collinear ‘knock-on’ provides insight into the reactive collision-complex, which lasts for approximately a million-millionth of a second. “Our results clearly tell us that the transition state at the top of the energy barrier lasts for so little time that it cannot fully scramble its momenta. Instead, it remembers the direction from which the attacking fluorine atom came.”
In the 1930s, chemists began calculating the likelihood of forming a transition state on the assumption that it scrambles its energy, like a hot molecule. Although it was an assumption, it appeared well-established and gave rise to the statistical “transition state theory” of reaction rates. This is still the favored method for calculating reaction rates.
“Now, with the ability to observe the reagents and the products at the molecular level, one can see precisely how the reagents approach and subsequently how the products separate,” Polanyi says. “But this runs contrary to the classic 90-year old statistical model. If the energy and momentum were randomized in the hot transition state, the products would not exhibit a clear memory of the direction of approach of the reagents. Energy-randomization would work to erase that memory.”
The researchers say the observed directional motion of the reaction products favours a deterministic model of the transition state to replace the long-standing statistical model. Additionally, the observed reaction dynamics allow the reagent energy to be passed on in successive collinear collisions.
###
The research was funded in part by the Natural Sciences and Engineering Research Council of Canada (NSERC) and the University of Toronto NSERC General Research Fund. Theoretical calculations were performed on the Niagara cluster at SciNet HPC Consortium.
MEDIA CONTACTS:
John Polanyi
Department of Chemistry
University of Toronto
[email protected]
Sean Bettam
Communications + Public Affairs, Faculty of Arts & Science
University of Toronto
[email protected]
Media Contact
Sean Bettam
[email protected]
Related Journal Article
http://dx.